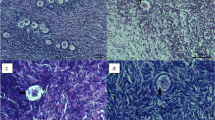
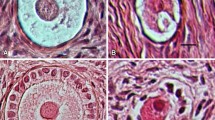
Abstract
Purpose
To evaluate the efficiency of an original slow freezing protocol on the quality and function of human ovarian cortex.
Methods
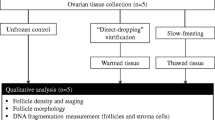
Human ovarian tissues were cryopreserved using a freezing medium supplemented with propanediol and raffinose as cryoprotectants and antioxidants (L-glutamine, taurine). Samples were then frozen using a faster cooling rate than the usual one. Viability and morphology of follicles, DNA fragmentation in follicles and stroma as well as histology of the vascular endothelium were analyzed before and after freezing/thawing. Moreover, a functional analysis was performed based on the evaluation of follicular growth and development in thawed ovarian tissues that were cultured in vitro.
Results
Our freezing/thawing protocol allows preservation of a high proportion of viable follicles and the preservation of the different follicle developmental stages (p > 0.05 versus fresh control). 70.5 ± 5.2 % of follicles retained an intact morphology after cryopreservation (p = 0.04). Stroma cells but not follicles exhibited a slight increase of DNA fragmentation after thawing (p < 0.05). Microvessel endothelium within thawed tissues appeared to be preserved. Granulosa cells showed signs of proliferation in follicles cultured for 12 days. Secretion of 17β-oestradiol significantly increased during in vitro culture.
Conclusions
This protocol leads to good preservation of ovarian integrity and functionality post-thawing and thus appears as a suitable technique of ovarian tissue cryopreservation in clinical settings. Further research could be extended to optimize conditions of in vitro culture.



Similar content being viewed by others
References
Abir R, Orvieto R, Raanani H, Feldberg D, Nitke S, Fisch B. Parameters affecting successful transplantation of frozen-thawed human fetal ovaries into immunodeficient mice. Fertil Steril. 2003;80(2):421–8.
Boland NI, Humpherson PG, Leese HJ, Gosden RG. Pattern of lactate production and steroidogenesis during growth and maturation of mouse ovarian follicles in vitro. Biol Reprod. 1993;48(4):798–806.
Borini A, Bonu MA, Coticchio G, Bianchi V, Cattoli M, Flamigni C. Pregnancies and births after oocyte cryopreservation. Fertil Steril. 2004;82(3):601–5. doi:10.1016/j.fertnstert.2004.04.025.
Cabrita E, Ma S, Diogo P, Martinez-Paramo S, Sarasquete C, Dinis MT. The influence of certain aminoacids and vitamins on post-thaw fish sperm motility, viability and DNA fragmentation. Anim Reprod Sci. 2011;125(1–4):189–95. doi:10.1016/j.anireprosci.2011.03.003.
Courbiere B, Massardier J, Salle B, Mazoyer C, Guerin JF, Lornage J. Follicular viability and histological assessment after cryopreservation of whole sheep ovaries with vascular pedicle by vitrification. Fertil Steril. 2005;84 Suppl 2:1065–71. doi:10.1016/j.fertnstert.2005.03.079.
Demirci B, Lornage J, Salle B, Frappart L, Franck M, Guerin JF. Follicular viability and morphology of sheep ovaries after exposure to cryoprotectant and cryopreservation with different freezing protocols. Fertil Steril. 2001;75(4):754–62.
Demirci B, Salle B, Frappart L, Franck M, Guerin JF, Lornage J. Morphological alterations and DNA fragmentation in oocytes from primordial and primary follicles after freezing-thawing of ovarian cortex in sheep. Fertil Steril. 2002;77(3):595–600.
Ding CC, Thong KJ, Krishna A, Telfer EE. Activin A inhibits activation of human primordial follicles in vitro. J Assist Reprod Genet. 2010;27(4):141–7. doi:10.1007/s10815-010-9395-6.
Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, et al. Children born after autotransplantation of cryopreserved ovarian tissue. a review of 13 live births. Ann Med. 2011;43(6):437–50. doi:10.3109/07853890.2010.546807.
Eroglu A. Cryopreservation of mammalian oocytes by using sugars: intra- and extracellular raffinose with small amounts of dimethylsulfoxide yields high cryosurvival, fertilization, and development rates. Cryobiology. 2010;60(3 Suppl):S54–9. doi:10.1016/j.cryobiol.2009.07.001.
Fabbri R, Pasquinelli G, Keane D, Magnani V, Paradisi R, Venturoli S. Optimization of protocols for human ovarian tissue cryopreservation with sucrose, 1,2-propanediol and human serum. Reprod BioMed Online. 2010;21(6):819–28. doi:10.1016/j.rbmo.2010.07.008.
Fauque P, Ben Amor A, Joanne C, Agnani G, Bresson JL, Roux C. Use of trypan blue staining to assess the quality of ovarian cryopreservation. Fertil Steril. 2007;87(5):1200–7. doi:10.1016/j.fertnstert.2006.08.115.
Gidoni Y, Holzer H, Tulandi T, Tan SL. Fertility preservation in patients with non-oncological conditions. Reprod Biomed Online. 2008;16(6):792–800.
Gook DA, Edgar DH, Stern C. Effect of cooling rate and dehydration regimen on the histological appearance of human ovarian cortex following cryopreservation in 1, 2-propanediol. Hum Reprod. 1999;14(8):2061–8.
Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at −196 degrees C. Hum Reprod. 1994;9(4):597–603.
Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod. 1986;1(2):81–7.
Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17(2):121–55.
Hardikar AA, Risbud MV, Remacle C, Reusens B, Hoet JJ, Bhonde RR. Islet cryopreservation: improved recovery following taurine pretreatment. Cell Transplant. 2001;10(3):247–53.
Hovatta O, Silye R, Krausz T, Abir R, Margara R, Trew G, et al. Cryopreservation of human ovarian tissue using dimethylsulphoxide and propanediol-sucrose as cryoprotectants. Hum Reprod. 1996;11(6):1268–72.
Hreinsson JG, Scott JE, Rasmussen C, Swahn ML, Hsueh AJ, Hovatta O. Growth differentiation factor-9 promotes the growth, development, and survival of human ovarian follicles in organ culture. J Clin Endocrinol Metab. 2002;87(1):316–21.
Isachenko V, Lapidus I, Isachenko E, Krivokharchenko A, Kreienberg R, Woriedh M, et al. Human ovarian tissue vitrification versus conventional freezing: morphological, endocrinological, and molecular biological evaluation. Reproduction. 2009;138(2):319–27. doi:10.1530/REP-09-0039.
Jain A, Mohanka R, Orloff M, Abt P, Kashyap R, Cullen J, et al. University of Wisconsin versus histidine-tryptophan-ketoglutarate for tissue preservation in live-donor liver transplantation. Exp Clin Transplant. 2006;4(1):451–7.
Kedem A, Fisch B, Garor R, Ben-Zaken A, Gizunterman T, Felz C, et al. Growth differentiating factor 9 (GDF9) and bone morphogenetic protein 15 both activate development of human primordial follicles in vitro, with seemingly more beneficial effects of GDF9. J Clin Endocrinol Metab. 2011;96(8):E1246–54. doi:10.1210/jc.2011-0410.
Kedem A, Hourvitz A, Fisch B, Shachar M, Cohen S, Ben-Haroush A, et al. Alginate scaffold for organ culture of cryopreserved-thawed human ovarian cortical follicles. J Assist Reprod Genet. 2011;28(9):761–9. doi:10.1007/s10815-011-9605-x.
Keros V, Xella S, Hultenby K, Pettersson K, Sheikhi M, Volpe A, et al. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod. 2009;24(7):1670–83. doi:10.1093/humrep/dep079.
Konc J, Kanyo K, Varga E, Kriston R, Cseh S. Births resulting from oocyte cryopreservation using a slow freezing protocol with propanediol and sucrose. Syst Biol Reprod Med. 2008;54(4–5):205–10. doi:10.1080/19396360802415778.
Louhio H, Hovatta O, Sjoberg J, Tuuri T. The effects of insulin, and insulin-like growth factors I and II on human ovarian follicles in long-term culture. Mol Hum Reprod. 2000;6(8):694–8.
Marsella T, Sena P, Xella S, La Marca A, Giulini S, De Pol A, et al. Human ovarian tissue cryopreservation: effect of sucrose concentration on morphological features after thawing. Reprod Biomed Online. 2008;16(2):257–67.
Muhlbacher F, Langer F, Mittermayer C. Preservation solutions for transplantation. Transplant Proc. 1999;31(5):2069–70.
Nisolle M, Casanas-Roux F, Qu J, Motta P, Donnez J. Histologic and ultrastructural evaluation of fresh and frozen-thawed human ovarian xenografts in nude mice. Fertil Steril. 2000;74(1):122–9.
Oktem O, Urman B. Options of fertility preservation in female cancer patients. Obstet Gynecol Surv. 2010;65(8):531–42. doi:10.1097/OGX.0b013e3181f8c0aa.
Poirot C, Schubert B. Fertility preservation in prepubertal children. Bull Cancer. 2011;98(5):489–99. doi:10.1684/bdc.2011.1362.
Rimon E, Cohen T, Dantes A, Hirsh L, Amit A, Lessing JB, et al. Apoptosis in cryopreserved human ovarian tissue obtained from cancer patients: a tool for evaluating cryopreservation utility. Int J Oncol. 2005;27(2):345–53.
Sadeu JC, Smitz J. Growth differentiation factor-9 and anti-Mullerian hormone expression in cultured human follicles from frozen-thawed ovarian tissue. Reprod Biomed Online. 2008;17(4):537–48.
Sanchez F, Romero S, Albuz FK, Smitz J. In vitro follicle growth under non-attachment conditions and decreased FSH levels reduces Lhcgr expression in cumulus cells and promotes oocyte developmental competence. J Assist Reprod Genet. 2012;29(2):141–52. doi:10.1007/s10815-011-9690-x.
Sanfilippo S, Canis M, Ouchchane L, Botchorishvili R, Artonne C, Janny L, et al. Viability assessment of fresh and frozen/thawed isolated human follicles: reliability of two methods (Trypan blue and Calcein AM/ethidium homodimer-1). J Assist Reprod Genet. 2011;28(12):1151–6. doi:10.1007/s10815-011-9649-y.
Schmidt KT, Larsen EC, Andersen CY, Andersen AN. Risk of ovarian failure and fertility preserving methods in girls and adolescents with a malignant disease. BJOG. 2010;117(2):163–74. doi:10.1111/j.1471-0528.2009.02408.x.
Schubert B, Canis M, Darcha C, Artonne C, Pouly JL, Dechelotte P, et al. Human ovarian tissue from cortex surrounding benign cysts: a model to study ovarian tissue cryopreservation. Hum Reprod. 2005;20(7):1786–92. doi:10.1093/humrep/dei002.
Schubert B, Canis M, Darcha C, Artonne C, Smitz J, Grizard G. Follicular growth and estradiol follow-up after subcutaneous xenografting of fresh and cryopreserved human ovarian tissue. Fertil Steril. 2008;89(6):1787–94. doi:10.1016/j.fertnstert.2007.03.101.
Shiva Shankar Reddy N, Jagan Mohanarao G, Atreja SK. Effects of adding taurine and trehalose to a tris-based egg yolk extender on buffalo (Bubalus bubalis) sperm quality following cryopreservation. Anim Reprod Sci. 2010;119(3–4):183–90. doi:10.1016/j.anireprosci.2010.01.012.
Silber SJ. Ovary cryopreservation and transplantation for fertility preservation. Mol Hum Reprod. 2012;18(2):59–67. doi:10.1093/molehr/gar082.
Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, et al. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update. 2010;16(4):395–414. doi:10.1093/humupd/dmp056.
Sordet O, Khan QA, Pommier Y. Apoptotic topoisomerase I-DNA complexes induced by oxygen radicals and mitochondrial dysfunction. Cell Cycle. 2004;3(9):1095–7.
Tjer GC, Chiu TT, Cheung LP, Lok IH, Haines CJ. Birth of a healthy baby after transfer of blastocysts derived from cryopreserved human oocytes fertilized with frozen spermatozoa. Fertil Steril. 2005;83(5):1547–9. doi:10.1016/j.fertnstert.2005.01.007.
Touraine P, Beau I, Gougeon A, Meduri G, Desroches A, Pichard C, et al. New natural inactivating mutations of the follicle-stimulating hormone receptor: correlations between receptor function and phenotype. Mol Endocrinol. 1999;13(11):1844–54.
Weissman A, Gotlieb L, Colgan T, Jurisicova A, Greenblatt EM, Casper RF. Preliminary experience with subcutaneous human ovarian cortex transplantation in the NOD-SCID mouse. Biol Reprod. 1999;60(6):1462–7.
Woodruff TK, Shea LD. The role of the extracellular matrix in ovarian follicle development. Reprod Sci. 2007;14(8 Suppl):6–10. doi:10.1177/1933719107309818.
Xiao Z, Wang Y, Li L, Li SW. Cryopreservation of the human ovarian tissue induces the expression of Fas system in morphologically normal primordial follicles. Cryo-Letters. 2010;31(2):112–9.
Xiao Z, Wang Y, Li L, Luo S, Li SW. Needle immersed vitrification can lower the concentration of cryoprotectant in human ovarian tissue cryopreservation. Fertil Steril. 2010;94(6):2323–8. doi:10.1016/j.fertnstert.2010.01.011.
Xiong Y, Connolly T, Futcher B, Beach D. Human D-type cyclin. Cell. 1991;65(4):691–9.
Zheng JH, Min ZL, Li YL, Zhu YH, Ye TJ, Li JQ, et al. A modified CZ-1 preserving solution for organ transplantation: comparative study with UW preserving solution. Chin Med J (Engl). 2008;121(10):904–9.
Acknowledgments
We would like to thank all the surgical team in the department of gynecology, CHU Estaing, Clermont-Ferrand (France) for their help to recruit patients. We are grateful to Dr Wassim Essamet for his expertise regarding the histology, Emmanuel Bourgeois and Christine Artonne for their precious technical assistance and Mrs Elizabeth Petit for language revision of the manuscript. Thanks are also due to the women who donated tissue for this research.
This work is supported by an industrial PhD fellowship (Convention Industrielle de Formation par la Recherche, CIFRE) with the Centre International de Chirurgie Endoscopique (CICE), France (Grant No: 176/2009).
Conflicts of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule An original slow freezing protocol able to protect both quality and functionality of human ovarian tissue, and easily applicable in a clinical setting.
Rights and permissions
About this article
Cite this article
Sanfilippo, S., Canis, M., Romero, S. et al. Quality and functionality of human ovarian tissue after cryopreservation using an original slow freezing procedure. J Assist Reprod Genet 30, 25–34 (2013). https://doi.org/10.1007/s10815-012-9917-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-012-9917-5