Abstract
Purpose
The present study was aimed to find out whether postovulatory aging-induced abortive spontaneous egg activation (SEA) is due to insufficient increase of cytosolic free Ca2+ level.
Methods
Immature female rats (22–24 days old) were subjected to superovulation induction protocol. Eggs were collected 14, 17 and 19 h post-hCG surge to induce in vivo egg aging. The eggs were collected 14 h post-hCG surge and cultured in vitro for 3, 5 and 7 h to induce in vitro egg aging. The morphological changes, rate of abortive SEA, chromosomal status and cytosolic free Ca2+ levels were analyzed.
Results
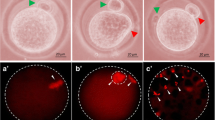
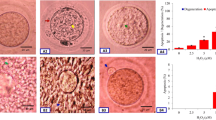
Postovulatory aging induced morphological features characteristics of abortive SEA in a time-dependent manner in vivo as well as in vitro. The extracellular Ca2+ increased rate of abortive SEA during initial period of culture, while co-addition of a nifedipine (L-type Ca2+ channel blocker) protected against postovulatory aging-induced abortive SEA. However, CI induced morphological features characteristics of egg activation (EA) in a dose-dependent manner. As compare to control, an increase of cytosolic free Ca2+ level (1.42 times) induced abortive SEA, while further increase of cytosolic free Ca2+ level (2.55 times) induced EA.
Conclusion
Our results show that an insufficient cytosolic free Ca2+ level is associated with postovulatory aging -induced abortive SEA, while furthermore increase is required to induce EA in rat.








Similar content being viewed by others
References
Schultz RM, Kopf GS. Molecular basis of mammalian egg activation. Curr Top Dev Biol. 1995;30:21–62.
Xu Z, Abbott A, Kopf GS, Schultz RM, Ducibella T. Spontaneous activation of ovulated mouse eggs: time-dependent effects on M-phase exit, cortical granule exocytosis, maternal messenger ribonucleic acid recruitment, and Inositol 1,4,5-trisphosphate sensitivity. Biol Reprod. 1997;57:743–50.
Carroll J, Swann K. Spontaneous cytosolic calcium oscillations driven by inositol trisphosphate occur during in vitro maturation of mouse oocytes. J Biol Chem. 1992;267:11196–201.
Miyazaki S, Yuzaki M, Nakada K, Shirakawa H, Nakanishi S, Nakade S, et al. Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science. 1992;257:251–5.
Ciapa B, Chiri S. Egg activation: Upstream of the fertilization calcium signal. Biol Cell. 2000;92:215–33.
Vincent C, Cheek TR, Johnson MH. Cell cycle progression of parthenogenetically activated mouse oocytes to interphase is dependent on the level of internal calcium. J Cell Sci. 1992;103:389–96.
Liu CT, Chen CH, Cheng SP, Ju JC. Parthenogenesis of rabbit oocytes activated by different stimuli. Anim Reprod Sci. 2002;70:267–76.
Ito J, Shimada M. Timing of MAP kinase inactivation effects on emission of polar body in porcine oocytes activated by calcium ionophore. Mol Reprod Dev. 2005;70:64–9.
Jilek F, Huttelova R, Petr J, Holubova M, Rozinek J. Activation of pig oocytes using calcium ionophore: effect of protein synthesis inhibitor cyclohexamide. Anim Reprod Sci. 2000;63:101–11.
Ruddock NT, Machaty Z, Cabot RA, Prather RS. Porcine oocyte activation: roles of calcium and pH. Mol Reprod Dev. 2001;59:227–34.
Ito J, Shimada M, Terada T. Effect of protein kinase C inhibitor on mitogen-activated protein kinase and p34cdc2 kinase activity during parthenogenetic activation of porcine oocytes by calcium ionophore. Biol Reprod. 2003;69:1675–82.
Sergeev IN, Norman AV. Calcium as a mediator of apoptosis in bovine oocytes and preimplantation embryos. Endocrine. 2003;22:169–76.
Ito J, Shimada M, Terada T. Mitogen-activated protein kinase kinase inhibitor suppresses cyclin B1 synthesis and reactivation of p34cdc2 kinase, which improves pronuclear formation rate in matured porcine oocytes activated by Ca2+ ionophore. Biol Reprod. 2004;70:797–04.
Lu Q, Chen ZJ, Gao X, Ma SY, Li M, Hu JM, et al. Oocyte activation with calcium ionophore A23187 and puromycin on human oocytes that failed to fertilize after intracytoplasmicsperm injection. Zhonghua Fu Chan Ke Za Zhi. 2006;41:182–5.
Ross PJ, Yabuuchi A, Cibelli JB. Oocyte spontaneous activation in different rat strains. Cloning Stem Cells. 2006;8:275–82.
Chaube SK, Dubey PK, Mishra SK, Shrivastav TG. Verapamil reversibly inhibits spontaneous parthenogenetic activation in aged rat eggs cultured in vitro. Cloning Stem Cells. 2007;9:608–17.
Fukuda A, Roudebush WE, Thatcher SS. Influences of in vitro oocyte aging on microfertilization in the mouse with reference to zona hardening. J Assist Reprod Genet. 1992;9:378–83.
Yanagida K, Yazawa H, Katayose H, Suzuki K, Hoshi K, Sato A. Influence of oocyte preincubation time on fertilization after intracytoplasmic sperm injection. Hum Reprod. 1998;13:2223–6.
Hayes E, Galea S, Verkuylen A, Pera M, Morrison J, Lacham-Kaplan O, et al. Nuclear transfer of adult and genetically modified fetal cells of the rat. Physiol Genom. 2001;5:193–04.
Zhou Q, Renard JP, Friec LG, Brochard V, Beaujean N, Cherifi Y, et al. Generation of fertile cloned rats by regulating oocyte activation. Science. 2003;302:1179.
Wu YG, Zhou P, Lan GC, Wang G, Luo MJ, Tan JH. The effects of delayed activation and MG132 treatment on nuclear remodeling and preimplantation development of embryos cloned by electrofusion are correlated with the age of recipient cytoplasts. Cloning Stem Cells. 2007;9:417–31.
Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2008;44:280–7.
Liu N, Wu YG, Lan GC, Sui HS, Ge L, Wang JZ, et al. Pyruvate prevents aging of mouse oocytes. Reproduction. 2009;138:223–34.
Galat V, Zhou Y, Taborn G, Garton R, Iannaccone P. Overcoming M-III arrest from spontaneous activation in cultured rat oocytes. Cloning Stem Cells. 2007;9:303–14.
Keefer CL, Schuetz AW. Spontaneous activation of ovulated rat oocytes during in vitro culture. J Exp Zool. 1982;224:371–7.
Kubiak JZ. Mouse oocytes gradually develop the capacity for activation during the metaphase II arrest. Develop Biol. 1989;136:537–45.
Zernika-Goetz M. Spontaneous and induced activation of rat oocytes. Mol Reprod Dev. 1991;28:169–76.
Cui W, Zhang J, Lian HY, Wang HL, Miao DQ, Zhang CX, et al. Roles of MAPK and spindle assembly checkpoint in spontaneous activation and M III arrest of rat oocytes. PLoS One. 2012;7:e32044.
Yoo JC, Smith LC. Extracellular calcium induces activation of Ca2+/calmodulin dependent protein kinase II and mediates spontaneous activation in rat oocytes. Biochem Biophys Res Commu. 2007;359:854–9.
Chebotareva T, Taylor J, Mullins JJ, Wilmut I. Rat eggs cannot wait: spontaneous exit from meiotic metaphase-II arrest. Mol Reprod Dev. 2011;78:798–07.
Ben-Yosef D, Oron Y, Shalgi R. Low temperature and fertilization-induced Ca2+ changes in rat eggs. Mol Reprod Dev. 1995;42:122–9.
Chaube SK, Khatun S, Mishra SK, Srivastav TG. Calcium ionophore-induced egg activation and apoptosis are associated with the generation of intracellular hydrogen peroxide. Free Radic Res. 2008;42:212–20.
Chaube SK, Tripathi A, Khatun S, Mishra SK, Prasad PV, Shrivastav TG. Extracellular calcium protects against verapamil-induced metaphase-II arrest and initiation of apoptosis in aged rat eggs. Cell Biol Int. 2009;33:337–43.
Gavet O, Pines J. Progressive activation of cyclin B1-cdk1 coordinates entry to mitosis. Develop Cell. 2010;18:533–43.
Tripathi A, Chaube SK. High cytosolic free calcium level signals apoptosis through mitochondria-caspase mediated pathway in rat eggs cultured in vitro. Apoptosis. 2012;17:439–48.
Homa ST, Carroll J, Swann K. The role of calcium in mammalian oocyte maturation and eggs activation. Hum Reprod. 1993;8:1274–81.
Tosti E. Calcium ion currents mediating oocyte maturation events. Reprod Biol Endocrinol. 2006;4:26–34.
Stricker SA. Comparative biology of calcium signaling during fertilization and egg activation in animals. Develop Biol. 1999;211:157–76.
Tripathi A, Premkumar KV, Chaube SK. Meiotic cell cycle arrest in mammalian oocytes. J Cell Physiol. 2010;223:592–00.
Borges E, PaesdeAlmeidaFerreiraBraga D, CarvalhodeSousaBonetti T, Laconelli Jr A, Franco Jr JG. Artificial oocyte activation with calcium ionophore A23187 in intracytoplasmic sperm injection cycles using surgically retrieved spermatozoa. Fertil Steril. 2009;92:131–36.
Terada Y, Hasegawa H, Takahashi A, Ugajin T, Yaegashi N, Okamura K. Successful pregnancy after oocyte activation by a calcium ionophore for a patient with recurrent intracytoplasmic sperm injection failure, with an assessment of oocyte activation and sperm centrosomal function using bovine eggs. Fertil Steril. 2009;91:e11–4.
Gualtieri R, Mollo V, Barbato V, Fiorentino I, Iaccarino M, Talevi R. Ultrastructure and intracellular calcium response during activation in vitrified and slow-frozen human oocytes. Hum Reprod. 2011;26:2452–60.
Acknowledgments
The authors are very thankful to Prof. T.G. Shrivastav, Department of Reproductive Biomedicine, National Institute of Health and Family Welfare, Baba Gang Nath Marg, New Delhi-110067, India for providing fluorescence light microscope facility (Nikon, Eclipse; E-80i, Japan).
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
Postovulatory aging induces insufficient increase of cytosolic free Ca2+ level leading to abortive spontaneous egg activation, while further increase of Ca2+ level is required to induce egg activation.
Rights and permissions
About this article
Cite this article
Premkumar, K.V., Chaube, S.K. An insufficient increase of cytosolic free calcium level results postovulatory aging-induced abortive spontaneous egg activation in rat. J Assist Reprod Genet 30, 117–123 (2013). https://doi.org/10.1007/s10815-012-9908-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-012-9908-6