Abstract
Purpose
To study the effect of addition of zinc to human semen sample prior to cryopreservation on post-thaw sperm quality and function.
Methods
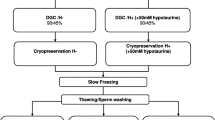
Semen samples were collected from men attending university infertility clinic for semen analysis (n=109). Liquefied semen samples were cryopreserved in glycerol-egg yolk- citrate medium with or without the prior addition of zinc (100 μM) and stored in liquid nitrogen. After 10 days, the semen samples were thawed to assess the outcome. Sperm motility, DNA integrity, mitochondrial potential and the ability of spermatozoa to undergo capacitation and acrosome reaction was assessed in post-thaw samples.
Results
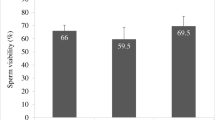
Semen samples cryopreserved after addition of zinc had a significantly higher percentage of sperm with intact DNA (p<0.001), mitochondrial function (p<0.001) and progressive motility (p<0.01) compared to the semen samples cryopreserved without zinc supplementation. Apart from this, ability to undergo capacitation and acrosome reaction in vitro was significantly higher in semen samples cryopreserved with zinc (p<0.0001 and p<0.001 respectively).
Conclusions
Addition of zinc to semen samples prior to cryopreservation helps in preventing the freeze-thaw-induced sperm DNA damage and loss of sperm function.




Similar content being viewed by others
References
Critser JK, Huse-Benda AR, Aaker DV, Arneson BW, Ball GD. Cryopreservation of human spermatozoa. The effect of cryoprotectants on motility. Fertil Steril. 1988;50:314–20.
Zribi N, Chakroun NF, EI Euch H, Gargouri J, Bahloul A, Keskes LA. Effects of cryopreservation on human sperm deoxyribonucleic acid integrity. Fertil Steril. 2010;93:159–66.
O’Connell M, McClure N, Lewis SE. The effect of cryopreservation on sperm morphology, motility and mitochondrial function. Hum Reprod. 2002;17:704–9.
Donnelly ET, McClure N, Lewis SEM. Cryopreservation of human semen and prepared sperm: effects on motility parameters and DNA integrity. Fertil Steril. 2001;76:892–900.
Donnelly ET, Steele KE, McClure N, Lewis SEM. Assessment of DNA integrity and morphology of ejaculated spermatozoa from fertile and infertile men before and after cryopreservation. Hum Reprod. 2001;16:1191–9.
Yoshida H, Hoshiai H, Fukaya T, Yajima A. Fertilization of fresh and frozen spermatozoa. Assist Reprod Technol Androl. 1990;1:164–72.
Bakos HW, Thompson JG, Feil D, Lane M. Sperm DNA damage is associated with assisted reproductive technology pregnancy. Int J Androl. 2008;31:518–26.
Thomson LK, Fleming SD, Aitken RJ, De Iuliis GN, Zieschang JA, Clark AM. Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum Reprod. 2009;24:2061–70.
Lopes S, Jurisicova A, Sun JG, Casper RF. Reactive oxygen species: potential cause for DNA fragmentation in human spermatozoa. Hum Reprod. 1998;13:896–900.
Twigg J, Fulton N, Gomez E, Irvine DS, Aitken RJ. Analysis of the impact of intracellular reactive oxygen species generation on the structural and functional integrity of the human spermatozoa: lipid peroxidation, DNA fragmentation and effectiveness of antioxidants. Hum Reprod. 1998;13:1429–36.
Duru NK, Morshedi M, Oehninger S. Effects of hydrogen peroxide on DNA and plasma membrane integrity of human spermatozoa. Fertil Steril. 2000;74:1200–7.
Branco CS, Garcez ME, Pasqualotto FF, Erdtman B, Salvador M. Resveratrol and ascorbic acid prevent DNA damage induced by cryopreservation in human semen. Cryobiology. 2010;60:235–7.
Kalthur G, Raj S, Thiyagarajan A, Kumar S, Kumar P, Adiga SK. Vitamin E supplementation in semen-freezing medium improves the motility and protects sperm from freeze-thaw-induced DNA damage. Fertil Steril. 2011;95:1149–51.
Kaji M. Zinc in endocrinology. Pediatr Int. 2001;16:1–7.
Wong WY, Merkus HM, Thomas CM, Menkveld R, Zielthuis GA, Steegers-Theunissen RP. Effect of folic acid and zinc sulphate on male factor sub fertility, a double blind, randomized placed controlled trial. Fertil Steril. 2002;77:491–8.
Suzuki T, Nakajima K, Yamamoto A, Yamanaka H. Metallothionein binding zinc inhibits nuclear chromatin decondensation of human spermatozoa. Andrologia. 1995;27:161–4.
Bjorndahl L, Kvist U. A model for the importance of zinc in the dynamics of human sperm chromatin stabilization after ejaculation in relation to sperm DNA vulnerability. Syst Biol Reprod Med. 2011;57:86–92.
World Health Organization. Laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambridge: Cambridge University Press; 1999.
Kalthur G, Adiga SK, Upadhya D, Rao S, Kumar P. Effect of cryopreservation on sperm DNA integrity in patients with teratospermia. Fertil Steril. 2008;89:1723–7.
Fernandez JL, Muriel L, Rivero MT, Goyanes V, Vazquez R, Alvarez JG. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl. 2003;24:59–66.
Johnson LV, Walsh ML, Chen LB. Localization of mitochondria in living cells by rhodamine 123. Proc Natl Acad Sci USA. 1980;77:990–4.
Colas C, Grasa P, Casao A, Gallego M, Abecia JA, Forcada F. Changes in calmodulin immunocytochemical localization associated with capacitation and acrosomal exocytosis of ram spermatozoa. Theriogenology. 2009;71:789–800.
Garde JJ, Ortiz N, García A, Gallego L. Use of a triple-stain technique to detect viability and acrosome reaction in deer spermatozoa. Arch Androl. 1997;39:1–9.
Chohan KR, Griffin JT, Carrell DT. Evaluation of chromatin integrity in human sperm using acridine orange staining with different fixatives and after cryopreservation. Andrologia. 2004;36:321–6.
Adiga SK, Khan Z, Upadhya D, Kalthur G, Kumar P. Ability of deoxyribonucleic acid-damaged sperm to withstand freeze-thaw-induced damage during cryopreservation. Fertil Steril. 2009;92:959–63.
Erenpreiss J, Elzanaty S, Giwercman A. Sperm DNA damage in men from infertile couples. Asian J Androl. 2008;10:786–90.
Hammadeh ME, Askari AS, Georg T, Rosenbaum P, Schmidt W. Effect of freeze–thawing procedure on chromatin stability, morphological alteration and membrane integrity of human spermatozoa in fertile and subfertile men. Int J Androl. 1999;22:155–62.
Varghese AC, Bragais FM, Mukhopadhyay D, Kundu S, Pal M, Bhattacharyya AK. Human sperm DNA integrity in normal and abnormal semen samples and its correlation with sperm characteristics. Andrologia. 2009;41:207–15.
Berg JM. Zinc fingers and other metal-binding domains. Elements for interactions between macromolecules. J Biol Chem. 1990;265:6513–6.
Balhorn R. The protamine family of sperm nuclear proteins. Genome Biol. 2007;8:227.
Kvist U, Bjorndahl L. Zinc preserves an inherent capacity for human sperm chromatin decondensation. Acta Physiol Scand. 1985;24:195–200.
Danscher G, Hammen R, Fjerdingstad E, Rebbe H. Zinc content of human ejaculate and the motility of sperm cells. Int J Androl 1978;1:1576–81.
Riffo M, Leiva S, Astudillo J. Effect of zinc on human sperm motility and the acrosome reaction. Int J Androl. 1992;15:229–37.
Stankovic H, Mikac-Devic D. Zinc and copper in human semen. Clin Chem Acta. 1976;70:123–6.
Caldamone AA, Freytag MK, Cockett AT. Seminal zinc and male infertility. Urology. 1979;13:280–1.
Calvin HI, Hwang FH-F, Wohlrab H. Localisation of zinc in a dense fiber-connecting piece fraction of rat sperm tails analogous chemically to hair keratin. Biol Reprod. 1975;13:228–39.
Bettger WJ, O’Dell BL. A critical physiological role of zinc in the structure and function of biomembranes. Life Sci. 1981;28:1425–38.
Kendall NR, McMullen S, Green A, Rodway RG. The effect of a zinc, cobalt and selenium soluble glass bolus on trace element status and semen quality of ram lambs. Anim Reprod Sci. 2000;62:277–83.
Andrews JC, Nolan JP, Hammerstedt RH, Bavister BD. Role of zinc during hamster sperm capacitation. Biol Reprod. 1994;51:1238–47.
Bilaspuri GS, Babbar BK. Effect of albumin and zinc on capacitation and acrosome reaction of buffalo spermatozoa. Indian J Anim Sci. 2007;77:688–92.
Liu DY, Sie BS, Liu ML, Agresta F, Baker HW. Relationship between seminal plasma zinc concentration and spermatozoa–zona pellucida binding and the ZP-induced acrosome reaction in sub fertile men. Asian J Androl. 2009;11:499–507.
Acknowledgments
Authors acknowledge financial assistance from Kasturba Medical College, Manipal, India, in the form of Postgraduate thesis grant sanctioned to APK (Grant No. PGR042). Technical support from Ms. Jayalaxmi Pai and Ms. Kirthi Patil (Clinical Embryology, Kasturba Medical College, Manipal) and Ms. Gimna Rathesh (Division of Nutrition, St. John’s Research Institute, St. John’s National Academy of Health Sciences, Bangalore) is thankfully acknowledged. Authors thank Dr. Prashanth Naik, Mangalore University for critical editing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
Addition of zinc to liquefied ejaculate prior to cryopreservation using glycerol- egg yolk- citrate buffered medium protects spermatozoa from freeze-thaw-induced DNA damage and loss of sperm function.
Author contribution
APK, SK, KG and SRS performed the study (APK and SK had equal contribution)
GK designed the study and wrote the paper
PT, SKA and PK helped in analyzing the data and writing the paper
Rights and permissions
About this article
Cite this article
Kotdawala, A.P., Kumar, S., Salian, S.R. et al. Addition of zinc to human ejaculate prior to cryopreservation prevents freeze-thaw-induced DNA damage and preserves sperm function. J Assist Reprod Genet 29, 1447–1453 (2012). https://doi.org/10.1007/s10815-012-9894-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-012-9894-8