Abstract
Objective
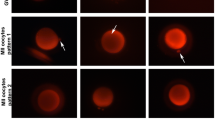
The aim of this study was to evaluate the impact of vitrification on mitochondrial membrane potential (ΔΨm) in human metaphase II (MII) oocytes, and the changes of ΔΨm on thawed MII oocytes.
Methods
MII oocytes were obtained from clinical IVF cycles when the oocytes were failed to fertilization within 24 h after insemination. All oocytes were randomly divided into 4 groups: non-frozen (fresh group), cultured for 0 h (0 h group), 2 h (2 h group) and 4 h (4 h group) after vitrification/thawing. All oocytes were stained with the ΔΨm-specific probe JC-1 and detected by laser scanning confocal microscope (LSCM) for mitochondrial analysis.
Results
The ΔΨm of oocytes was significantly decreased in 0 h and 2 h groups when compared with fresh group (0.93, 1.09 vs 1.34, P < 0.05), but similar between 4 h group and fresh group (1.30 vs 1.34, P > 0.05).
Conclusion
In the vitrification/thawing process, the ΔΨm of MII oocytes could have temporally dynamic changes within 2 h after thawing but would be fully recovered after 4 h culture.


Similar content being viewed by others
References
Ludwig M, Al-Hasani S, Felberbaum R, Diedrich K. New aspects of cryopreservation of oocytes and embryos in assisted reproduction. Hum Reprod. 1999;14(1):162–85.
Porcu E. Oocyte freezing. Semin Repord Med. 2001;19(3):221–30.
Borini A, Lagalla C, Bonu MA, Bianchi V, Flamigni C, Coticchio G. Cumulative pregnancy rates resulting from the use of fresh and frozen oocytes: 7 years’ experience. Reprod Biomed Online. 2006;12(4):481–6.
Selman H, Angelini A, Barnochi N, Brusco GF, Pacchiarotti A, Aragona C. Ongoing pregnancies after vitrification of human oocytes using a cpmbined solution of ethylene glycol and dimethy sulfoxide. Fertil Steril. 2006;84(4):997–1000.
Cobo A, Diaz C. Clinical application of oocyte vitrification: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2011;96(2):277–85.
Trokoudes KM, Pavlides C, Zhang X. Comparison outcome of fresh and vitrified donor oocytes in an egg-sharing donation program. Fertil Steril. 2011;95(6):1996–2000.
Azambuja R, Petracco A, Okada L, Michelon J, Badalotti F, Badalotti M. Experience of freezing human oocytes using sodium-depleted media. Reprod Biomed Online. 2011;22(1):83–7.
Gualtieri R, Iaccarino M, Mollo V, Prisco M, Iaccarino S, Talevi R. Slow cooling of human oocytes: Ultrastructural injuries and apoptotic status. Fertil Steril. 2009;91(4):1023–34.
Reers M, Smiley S, Mottola-Hartshorn C, Chen A, Lin M, Chen L. Mitochondrial membrane potential monitored by JC-1 dye. In Methods in Enzymology. 1995;260:406–17.
Ye H, Huang GN, Zeng PH, Pei L. IVF/ICSI outcomes between cycles with luteal estradiol (E2) pre-treatment before GnRH antagonist protocol and standard long GnRH agonist protocol: a prospective and randomized study. J Assist Reprod Genet. 2009;26(2–3):105–11.
Chian RC, Kuwayama M, Tan L, Tan J, Kato O, Nagai T. High survival rate of bovine oocytes matured in vitro following vitrification. Reprod Dev. 2004;50(6):685–96.
Wilding M, Dale B, Marino M, di Matteo L, Alviggi C, Pisaturo ML, et al. Mitochondrial aggregation patterns and actvity in human oocytes and preimplantation embryos. Hum Reprod. 2001;16(5):909–17.
Jones A, Blerkom JV, Davis P, Andrew AT. Cryopreservation of metaphase II human oocytes effects mitochondrial membrane potential:implications for developmental competence. Hum Reprod. 2004;19(8):1861–6.
Nottola SA, Coticchio G, Sciajno R, Gambardella A, Maione M, Scaravelli G, et al. Ultrastructural markers of quality in human mature oocytes vitrified using cryoleaf and cryoloop. Reprod Biomed Online. 2009;19 Suppl 3:17–27.
Jansen RP. Germline passage of mitochondria: quantitative considerations and possible embryological sequelae. Hum Reprod. 2000;15 Suppl 2:112–28.
Acton B, Jurisicova A, Jurisica I, Lasper RF. Alterations in mitochondrial membrane potential during preimplantation stages of mouse and human development. Mol Hum Reprod. 2004;10(2):23–32.
Wilding M, Fiorentino A, De Simone ML, Infante V, De Matteo L, Marino M, et al. Energy substrates, mitochondrial membrane potential and human preimplantation embryo division. Reprod BioMed Online. 2002;5(1):39–42.
Wilding M, De Placido G, De Matteo L, Marino M, Alviggi C, Dale B. Chaotic mosaicism in human preimplantation embryos is correlated with a low mitochondrial membrane potential. Fertil Steril. 2003;79(2):340–6.
Mitchell P, Moyle. Chemiosmotic hypothesis of oxidative Phosphorylation. Nature. 1967;213(5072):137–9.
Boiso I, Martí M, Santaló J, Ponsá M, Barri PN, Veiga A. A confocal microscopy analysis of the spindle and chromosome configurations of human oocytes cryopreserved at the germinal vesicle and metaphase II stage. Hum Reprod. 2002;17(7):1885–91.
Coticchio G, Borini A, Distratis V, Maione M, Scaravelli G, Bianchi V, et al. Qualitative and morphometric analysis of the ultrastructure of human oocytes cryopreserved by two alternative slow cooling protocols. Assist Reprod Genet. 2010;27(4):131–40.
Coticchio G, Bonu MA, Sciajno R, Sereni E, Bianchi V, Borini A. Truths and myths of oocyte sensitivity to controlled rate freezing. Reprod Biomed Online. 2007;15(1):24–30.
Ahn H, Sohn I, Kwon H, Jo D, Park Y, Min C. Characteristics of the cell membrane fluidity, actin fibers, and mitochondrial dysfunctions of frozen-thawed two-cell mouse embryos. Mol Reprod Dev. 2002;61(4):466–76.
Shaw JM, Kuleshova LL, MacFarlane DR, Trounson AO. Vitrification properties of solutions of ethylene glycol in saline containing PVP, Ficoll, or dextran. Cryobiology. 1997;35(3):219–29.
Kohaya N, Fujiwara K, Ito J, Kashiwazaki N. High developmental rates of mouse oocytes cryopreserved by an optimized vitrification protocol: the effects of cryoprotectants, calcium and cumulus cells. J Reprod Dev. 2011;57(6):675–80.
Kuleshova LL, MacFarlane DR, Trounson AO, Shaw JM. Sugars exert a major influence on the vitrification properties of ethylene glycol-based solutions and have low toxicity to embryos and oocytes. Cryobiology. 1999;38(2):119–30.
Bianchi V, Coticchio G, Fava L, Flamigni C, Borini A. Meiotic spindle imaging in human oocytes frozen with a slow freezing procedure involving high sucrose concentration. Hum Reprod. 2005;20(4):1078–83.
Chen SU, Lien YR, Chao KH, Ho HN, Yang YS, Lee TY. Effects of cryopreservation on meiotic spindles of oocytes and its dynamics after thawing: clinical implications in oocyte freezing–a review article. Mol Cell Endocrinol. 2003;202(1–2):101–7.
Manipalviratn S, Tong ZB, Stegmann B, Widra E, Carter J, DeCherney A. Effect of vitrification and thawing on human oocyte ATP concentration. Fertil Steril. 2011;95(5):1839–41.
Chen SU, Lien YR, Cheng YY, Chen HF, Ho HN, Yang YS. Vitrification of mouse oocytes using closed pulled straws (CPS) achieves a high survival and preserves good patterns of meiotic spindles, compared with conventional straws, open pulled straws (OPS) and grids. Hum Reprod. 2001;16(11):2350–6.
Porcu E, Fabbri R, Damiano G, Giunchi S, Fratto R, Ciotti PM, et al. Clinical experience and applications of oocyte cryopreservation. Mol Cell Endocrinol. 2000;169(1–2):33–7.
Acknowledgements
We greatly appreciate and thank Dr De-Yi Liu from the Melbourne IVF and the University of Melbourne in Australia, for his comments and revision of the final draft of manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule The article used human unfertilized metaphase II oocytes as material to detect the recovery ability of mitochondrial membrane potential (ΔΨm) after vitrification/thawing. The results showed that the ΔΨm of MII oocytes had temporally dynamic changes within 2h after thawing but could be fully recovered after 4 h culture.
Rights and permissions
About this article
Cite this article
Chen, C., Han, S., Liu, W. et al. Effect of vitrification on mitochondrial membrane potential in human metaphase II oocytes. J Assist Reprod Genet 29, 1045–1050 (2012). https://doi.org/10.1007/s10815-012-9848-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-012-9848-1