Abstract
Purpose
To study the effect of supplementing biotin to sperm preparation medium on the motility of frozen-thawed spermatozoa.
Methods
Semen samples of men attending the University infertility clinic (n = 105) were cryopreserved using glycerol-egg yolk-citrate buffered cryoprotective medium in liquid nitrogen. After a period of two weeks, the semen samples were thawed and the motile spermatozoa were extracted by swim-up technique using Earle’s balanced salt solution (EBSS) medium supplemented with either biotin (10 nM) or pentoxifylline (1 mM). The post-wash motility was observed up to 4 h after incubation.
Results
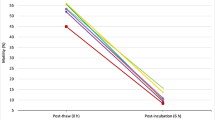
Both biotin and pentoxifylline supplementation resulted in significant increase in total motility (p < 0.05), progressive motility (p < 0.001) and rapid progressive motility (p < 0.05 v/s biotin and p < 0.01 v/s pentoxifylline) compared to the control at 1 h post-incubation period. Significantly higher percentage of total (p < 0.01, p < 0.05 in biotin and pentoxifylline respectively), progressive (p < 0.001) and rapid progressive motilities (p < 0.01) were observed in these two groups even at 2 h compared to the control. In the control group at 4 h after incubation, ~11% decline in total motility and ~8% decline in progressive motility was observed. However, in both biotin and pentoxifylline group the motility was significantly higher than control (p < 0.001). No significant difference in the motility was observed between biotin and pentoxifylline groups at any of the time intervals studied.
Conclusions
Biotin can enhance the sperm motility and prolong the survival of frozen-thawed semen samples which may have potential benefit in assisted reproductive technology field.



Similar content being viewed by others
References
Stanic P, Sonicki Z, Suchanek E. Effect of pentoxifylline on motility and membrane integrity of human cryopreserved spermatozoa. Int J Androl. 2002;25:186–90.
Tournaye H, Van der Linden M, Van den Abbeel E, Devroey P, Van Steirteghem A. Effects of pentoxifylline on in-vitro development of preimplantation mouse embryos. Hum Reprod. 1993;8:1475–80.
Tournaye H, Van der Linden M, Van den Abbeel E, Devroey P, Van Steirteghem A. Mouse in vitro fertilization using sperm treated with pentoxifylline and 2-deoxyadenosine. Fertil Steril. 1994;62:644–7.
Scott L, Smith S. Human sperm motility enhancing agents have detrimental effect on mouse oocytes and embryos. Fertil Steril. 1995;63:166–75.
Mock DM. Marginal biotin deficiency is common in normal human pregnancy and is highly teratogenic in mice. J Nutr. 2009;139:154–7.
Watanabe T. Dietary biotin deficiency affects reproductive function and prenatal development in hamsters. J Nutr. 1993;123:2101–8.
World Health Organization. Laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambridge: Cambridge University Press; 1999.
Kovacic B, Vlaisavljevic V, Reljic M. Clinical use of pentoxifylline for activation of immotile testicular sperm before ICSI in patients with non-obstructive azoospermia. J Androl. 2006;27:45–52.
Kalthur G, Adiga SK, Upadhya D, Rao S, Kumar P. Effect of cryopreservation on sperm DNA integrity in patients with teratospermia. Fertil Steril. 2008;89:1723–7.
Mahadevan M, Baker G. Assessment and preparation of semen for in vitro fertilization. In: Wood C, Trounson A, editors. Clinical in vitro fertilization. Berlin: Springer; 83. p. 97–1984.
Critser JK, Huse-Benda AR, Aaker DV, Arneson BW, Ball GD. Cryopreservation of human spermatozoa. The effect of cryoprotectants on motility. Fertil Steril. 1988;50:314–20.
Kalthur G, Raj S, Thiyagarajan A, Kumar S, Kumar P, Adiga SK. Vitamin E supplementation in semen cryopreservation medium improves the motility and protects sperm from freeze-thaw-induced DNA damage. Fertil Steril. 2011;95:1149–51.
Yovich JM, Edirisinghe WR, Cummins JM, Yovich JL. Influence of pentoxifylline in severe male factor infertility. Fertil Steril. 1990;53:715–22.
Yovich JL. Pentoxifylline: actions and applications in assisted reproduction. Hum Reprod. 1993;8:1786–91.
Yunes R, Fernadez P, Doncel GF, Acosta AA. Cyclin nucleotide phosphodiesterase inhibition increases tyrosine phosphorylation and hyper motility in normal and pathological human spermatozoa. Biocell. 2005;29:287–93.
Gil MA, Hernandez M, Roca J, et al. Pentoxifylline added to freezing or post-thaw extenders does not improve the survival or in vitro fertilizing ability of boar spermatozoa. Reproduction. 2010;139:557–64.
Centola GM, Cartie RJ, Cox C. Differential responses of human sperm to varying concentrations of pentoxifylline with demonstration of toxicity. J Androl. 1995;16:136–42.
Tash JS, Means AR. Cyclic adenosine 3′,5′ monophosphate, calcium and protein phosphorylation in flagellar motility. Biol Reprod. 1983;28:75–104.
Karl PI, Fisher SE. Biotin transport in microvillous membrane vesicles, cultured trophoblasts and the isolated perfused cotyledon of the human placenta. Am J Physiol. 1992;262:C302–8.
Schenker S, Hu Z, Johnson RF, Yang Y, Frosto T, Elliott BD, Henderson GI, Mock DM. Human placental biotin transport: normal characteristics and effect of ethanol. Alcohol Clin Exp Res. 1993;17:566–75.
Feldman G, Wolf B. Deficient acetyl CoA carboxylase activity in multiple carboxylase deficiency. Clin Chim Acta. 1981;111:147–51.
Packman S, Caswell N, Gonzales-Rios M, Kadlecek T, Cann H, Rassin D, McKay C. Acetyl CoA carboxylase in cultured fibroblasts: differential biotin dependence in the two types of biotin-responsive multiple carboxylase deficiency. Am J Hum Genet. 1984;36:80–92.
Kuroishi T, Rios-Avila L, Pestinger V, Wijeratne SS, Zempleni J. Biotinylation is a natural, albeit rare, modification of human histones. Mol Genet Metab. 2011;104:537–45.
Atamna H, Newberry J, Erlitzki R, Schultz CS, Ames BN. Biotin deficiency inhibits heme synthesis and impairs mitochondria in human lung fibroblasts. J Nutr. 2007;137:25–30.
Zempleni J, Teixeira DC, Kuroishi T, Cordonier EL, Baier S. Biotin requirements for DNA damage prevention. Mutat Res 2011;doi:mrfmmm2011.08.001/mrfmmm 2011.08.001.
Zempleni J, Mock DM. Marginal biotin deficiency is teratogenic. Proc Soc Exp Biol Med. 2000;223:14–21.
Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–5.
Acknowledgments
Authors kindly acknowledge the technical support provided by Mrs. Jalyalaxmi Pai, Mrs. Kirthi Patil, and Ms. Anita Poojary. Authors thank Dr. Prashanth Naik and Mr. Naveenchandra Kumar, Mangalore University for editing the language of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
Biotin supplementation to sperm wash medium can enhance the sperm motility and prolong the sperm survival.
Rights and permissions
About this article
Cite this article
Kalthur, G., Salian, S.R., Keyvanifard, F. et al. Supplementation of biotin to sperm preparation medium increases the motility and longevity in cryopreserved human spermatozoa. J Assist Reprod Genet 29, 631–635 (2012). https://doi.org/10.1007/s10815-012-9760-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-012-9760-8