Abstract
Purpose
To determine if the antioxidant superoxide dismutase-1 (SOD1 or Cu,Zn-SOD) is released by cultured human cleavage-stage embryos and to assess any link between SOD1 and implantation potential.
Methods
Women (n = 91; ≤40 years old) undergoing IVF treatment with transfer of one or two 8-cell embryos that resulted in 0 or 100% implantation were included. Following individual embryo culture, spent medium samples (n = 122) were collected and levels of SOD1 protein were measured by an enzyme-linked immunosorbent assay. SOD1 detection and concentration in embryo spent medium were analyzed in relation to embryo fragmentation and symmetry scores, and implantation (viable fetus at >12 weeks).
Results
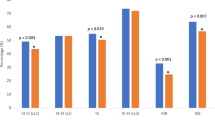
Cleavage-stage embryos release SOD1 protein into the spent culture medium. Neither detection nor concentration of SOD1 was related to implantation. There was a positive relationship between increased embryo fragmentation scores and SOD1 release, with no apparent association with symmetry. In non-pregnant cycles, the release of SOD1 decreased with increasing maternal age.
Conclusions
While SOD1 does not predict implantation potential of select good-quality embryos, our data support the need to evaluate the biological significance of released SOD1 by embryos of varying quality and from patients of varying age.


Similar content being viewed by others
References
Cummins JM, Breen TM, Harrison KL, Shaw JM, Wilson LM, Hennessey JF. A formula for scoring human embryo growth rates in in vitro fertilization: its value in predicting pregnancy and in comparison with visual estimates of embryo quality. J In Vitro Fert Embryo Transf. 1986;3:284–95.
Steer CV, Mills CL, Tan SL, Campbell S, Edwards RG. The cumulative embryo score: a predictive embryo scoring technique to select the optimal number of embryos to transfer in an in-vitro fertilization and embryo transfer programme. Hum Reprod. 1992;7:117–9.
Giorgetti C, Terriou P, Auquier P, Hans E, Spach JL, Salzmann J, Roulier R. Embryo score to predict implantation after in-vitro fertilization: based on 957 single embryo transfers. Hum Reprod. 1995;10:2427–31.
Holte J, Berglund L, Milton K, Garello C, Gennarelli G, Revelli A, Bergh T. Construction of an evidence-based integrated morphology cleavage embryo score for implantation potential of embryos scored and transferred on day 2 after oocyte retrieval. Hum Reprod. 2007;22:548–57.
Racowsky C, Ohno-Machado L, Kim J, Biggers JD. Is there an advantage in scoring early embryos on more than one day? Hum Reprod. 2009;24:2104–13.
Guerif F, Le Gouge A, Giraudeau B, Poindron J, Bidault R, Gasnier O, Royere D. Limited value of morphological assessment at days 1 and 2 to predict blastocyst development potential: a prospective study based on 4042 embryos. Hum Reprod. 2007;22:1973–81.
Katz-Jaffe MG, McReynolds S, Gardner DK, Schoolcraft WB. The role of proteomics in defining the human embryonic secretome. Mol Hum Reprod. 2009;15:271–7.
Seli E, Robert C, Sirard MA. OMICS in assisted reproduction: possibilities and pitfalls. Mol Hum Reprod. 2010;16:513–30.
Warner CM, Lampton PW, Newmark JA, Cohen J. Symposium: innovative techniques in human embryo viability assessment. Soluble human leukocyte antigen-G and pregnancy success. Reprod Biomed Online. 2008;17:470–85.
Nieder GL, Weitlauf HM, Suda-Hartman M. Synthesis and secretion of stage-specific proteins by peri-implantation mouse embryos. Biol Reprod. 1987;36:687–99.
Fridovich I. Oxygen toxicity: a radical explanation. J Exp Biol. 1998;201:1203–9.
Mondola P, Annella T, Santillo M, Santangelo F. Evidence for secretion of cytosolic CuZn superoxide dismutase by Hep G2 cells and human fibroblasts. Int J Biochem Cell Biol. 1996;28:677–81.
Mondola P, Annella T, Seru R, Santangelo F, Iossa S, Gioielli A, Santillo M. Secretion and increase of intracellular CuZn superoxide dismutase content in human neuroblastoma SK-N-BE cells subjected to oxidative stress. Brain Res Bull. 1998;45:517–20.
Cimini V, Ruggiero G, Buonomo T, Seru R, Sciorio S, Zanzi C, Santangelo F, Mondola P. CuZn-superoxide dismutase in human thymus: immunocytochemical localisation and secretion in thymus-derived epithelial and fibroblast cell lines. Histochem Cell Biol. 2002;118:163–9.
Scott R, Seli E, Miller K, Sakkas D, Scott K, Burns DH. Noninvasive metabolomic profiling of human embryo culture media using Raman spectroscopy predicts embryonic reproductive potential: a prospective blinded pilot study. Fertil Steril. 2008;90:77–83.
Seli E, Sakkas D, Scott R, Kwok SC, Rosendahl SM, Burns DH. Noninvasive metabolomic profiling of embryo culture media using Raman and near-infrared spectroscopy correlates with reproductive potential of embryos in women undergoing in vitro fertilization. Fertil Steril. 2007;88:1350–7.
Paszkowski T, Clarke RN. Antioxidative capacity of preimplantation embryo culture medium declines following the incubation of poor quality embryos. Hum Reprod. 1996;11:2493–5.
Bedaiwy M, Agarwal A, Said TM, Goldberg JM, Sharma RK, Worley S, Falcone T. Role of total antioxidant capacity in the differential growth of human embryos in vitro. Fertil Steril. 2006;86:304–9.
Bedaiwy MA, Falcone T, Mohamed MS, Aleem AA, Sharma RK, Worley SE, Thornton J, Agarwal A. Differential growth of human embryos in vitro: role of reactive oxygen species. Fertil Steril. 2004;82:593–600.
Bedaiwy MA, Mahfouz RZ, Goldberg JM, Sharma R, Falcone T. Abdel Hafez MF, Agarwal A: Relationship of reactive oxygen species levels in day 3 culture media to the outcome of in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil Steril. 2010;94:2037–42.
Wiener-Megnazi Z, Shiloh H, Avraham L, Lahav-Baratz S, Koifman M, Reznick AZ, Auslender R, Dirnfeld M. Oxidative parameters of embryo culture media may predict treatment outcome in in vitro fertilization: a novel applicable tool for improving embryo selection. Fertil Steril. 2011;95:979–84.
Reichman DE, Politch J, Ginsburg ES, Racowsky C. Extended in vitro maturation of immature oocytes from stimulated cycles: an analysis of fertilization potential, embryo development, and reproductive outcomes. J Assist Reprod Genet. 2010;27:347–56.
Racowsky C, Combelles CM, Nureddin A, Pan Y, Finn A, Miles L, Gale S, O'Leary T, Jackson KV. Day 3 and day 5 morphological predictors of embryo viability. Reprod Biomed Online. 2003;6:323–31.
Harvey MB, Arcellana-Panlilio MY, Zhang X, Schultz GA, Watson AJ. Expression of genes encoding antioxidant enzymes in preimplantation mouse and cow embryos and primary bovine oviduct cultures employed for embryo coculture. Biol Reprod. 1995;53:532–40.
Wrenzycki C, De Sousa P, Overstrom EW, Duby RT, Herrmann D, Watson AJ, Niemann H, O'Callaghan D, Boland MP. Effects of superovulated heifer diet type and quantity on relative mRNA abundances and pyruvate metabolism in recovered embryos. J Reprod Fertil. 2000;118:69–78.
Lequarre AS, Feugang JM, Malhomme O, Donnay I, Massip A, Dessy F, Van Langendonckt A. Expression of Cu/Zn and Mn superoxide dismutases during bovine embryo development: influence of in vitro culture. Mol Reprod Dev. 2001;58:45–53.
Santillo M, Secondo A, Seru R, Damiano S, Garbi C, Taverna E, Rosa P, Giovedi S, Benfenati F, Mondola P. Evidence of calcium- and SNARE-dependent release of CuZn superoxide dismutase from rat pituitary GH3 cells and synaptosomes in response to depolarization. J Neurochem. 2007;102:679–85.
Turner BJ, Atkin JD, Farg MA, Zang DW, Rembach A, Lopes EC, Patch JD, Hill AF, Cheema SS. Impaired extracellular secretion of mutant superoxide dismutase 1 associates with neurotoxicity in familial amyotrophic lateral sclerosis. J Neurosci. 2005;25:108–17.
Gomes C, Keller S, Altevogt P, Costa J. Evidence for secretion of Cu, Zn superoxide dismutase via exosomes from a cell model of amyotrophic lateral sclerosis. Neurosci Lett. 2007;428:43–6.
Mondola P, Ruggiero G, Seru R, Damiano S, Grimaldi S, Garbi C, Monda M, Greco D, Santillo M. The Cu, Zn superoxide dismutase in neuroblastoma SK-N-BE cells is exported by a microvesicles dependent pathway. Brain Res Mol Brain Res. 2003;110:45–51.
Goto Y, Noda Y, Mori T, Nakano M. Increased generation of reactive oxygen species in embryos cultured in vitro. Free Radic Biol Med. 1993;15:69–75.
Johnson MH, Nasr-Esfahani MH. Radical solutions and cultural problems: could free oxygen radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro? Bioessays. 1994;16:31–8.
Orsi NM, Leese HJ. Protection against reactive oxygen species during mouse preimplantation embryo development: role of EDTA, oxygen tension, catalase, superoxide dismutase and pyruvate. Mol Reprod Dev. 2001;59:44–53.
Betts DH, Madan P. Permanent embryo arrest: molecular and cellular concepts. Mol Hum Reprod. 2008;14:445–53.
Bain NT, Madan P, Betts DH. The early embryo response to intracellular reactive oxygen species is developmentally regulated. Reprod Fertil Dev. 2011;23:561–75.
Yang HW, Hwang KJ, Kwon HC, Kim HS, Choi KW, Oh KS. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum Reprod. 1998;13:998–1002.
Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–62.
Jurisicova A, Varmuza S, Casper RF. Programmed cell death and human embryo fragmentation. Mol Hum Reprod. 1996;2:93–8.
Hardy K. Apoptosis in the human embryo. Rev Reprod. 1999;4:125–34.
Kimura N, Tsunoda S, Iuchi Y, Abe H, Totsukawa K, Fujii J. Intrinsic oxidative stress causes either 2-cell arrest or cell death depending on developmental stage of the embryos from SOD1-deficient mice. Mol Hum Reprod. 2010;16:441–51.
Tarin JJ, Gomez-Piquer V, Pertusa JF, Hermenegildo C, Cano A. Association of female aging with decreased parthenogenetic activation, raised MPF, and MAPKs activities and reduced levels of glutathione S-transferases activity and thiols in mouse oocytes. Mol Reprod Dev. 2004;69:402–10.
Matos L, Stevenson D, Gomes F, Silva-Carvalho JL, Almeida H. Superoxide dismutase expression in human cumulus oophorus cells. Mol Hum Reprod. 2009;15:411–9.
Tatone C, Carbone MC, Falone S, Aimola P, Giardinelli A, Caserta D, Marci R, Pandolfi A, Ragnelli AM, Amicarelli F. Age-dependent changes in the expression of superoxide dismutases and catalase are associated with ultrastructural modifications in human granulosa cells. Mol Hum Reprod. 2006;12:655–60.
Carbone MC, Tatone C. Delle Monache S, Marci R, Caserta D, Colonna R, Amicarelli F: Antioxidant enzymatic defences in human follicular fluid: characterization and age-dependent changes. Mol Hum Reprod. 2003;9:639–43.
Tarin JJ. Potential effects of age-associated oxidative stress on mammalian oocytes/embryos. Mol Hum Reprod. 1996;2:717–24.
Tatone C, Amicarelli F, Carbone MC, Monteleone P, Caserta D, Marci R, Artini PG, Piomboni P, Focarelli R. Cellular and molecular aspects of ovarian follicle ageing. Hum Reprod Update. 2008;14:131–42.
Li J, Foote RH, Simkin M. Development of rabbit zygotes cultured in protein-free medium with catalase, taurine, or superoxide dismutase. Biol Reprod. 1993;49:33–7.
Nonogaki T, Noda Y, Narimoto K, Umaoka Y, Mori T. Protection from oxidative stress by thioredoxin and superoxide dismutase of mouse embryos fertilized in vitro. Hum Reprod. 1991;6:1305–10.
Nonogaki T, Noda Y, Narimoto K, Umaoka Y, Mori T. Effects of superoxide dismutase on mouse in vitro fertilization and embryo culture system. J Assist Reprod Genet. 1992;9:274–80.
Ali AA, Bilodeau JF, Sirard MA. Antioxidant requirements for bovine oocytes varies during in vitro maturation, fertilization and development. Theriogenology. 2003;59:939–49.
Liu Z, Foote RH. Development of bovine embryos in KSOM with added superoxide dismutase and taurine and with five and twenty percent O2. Biol Reprod. 1995;53:786–90.
Luvoni GC, Keskintepe L, Brackett BG. Improvement in bovine embryo production in vitro by glutathione-containing culture media. Mol Reprod Dev. 1996;43:437–43.
Thomas M, Jain S, Kumar GP, Laloraya M. A programmed oxyradical burst causes hatching of mouse blastocysts. J Cell Sci. 1997;110(Pt 14):1597–602.
Nasr-Esfahani MH, Aitken JR, Johnson MH. Hydrogen peroxide levels in mouse oocytes and early cleavage stage embryos developed in vitro or in vivo. Development. 1990;109:501–7.
Dalvit GC, Cetica PD, Pintos LN, Beconi MT. Reactive oxygen species in bovine embryo in vitro production. Biocell. 2005;29:209–12.
Lapointe J, Bilodeau JF. Antioxidant defenses are modulated in the cow oviduct during the estrous cycle. Biol Reprod. 2003;68:1157–64.
El Mouatassim S, Guerin P, Menezo Y. Mammalian oviduct and protection against free oxygen radicals: expression of genes encoding antioxidant enzymes in human and mouse. Eur J Obstet Gynecol Reprod Biol. 2000;89:1–6.
Guerin P, Menezo Y. Review: role of tubal environment in preimplantation embryogenesis: application to co-culture assays. Zygote. 2011;19:47–54.
Leyens G, Knoops B, Donnay I. Expression of peroxiredoxins in bovine oocytes and embryos produced in vitro. Mol Reprod Dev. 2004;69:243–51.
Harvey AJ, Kind KL, Thompson JG. REDOX regulation of early embryo development. Reproduction. 2002;123:479–86.
Dumollard R, Carroll J, Duchen MR, Campbell K, Swann K. Mitochondrial function and redox state in mammalian embryos. Semin Cell Dev Biol. 2009;20:346–53.
Braude P, Bolton V, Moore S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature. 1988;332:459–61.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
Release of the antioxidant superoxide dismutase-1 by human 8-cell embryos bears no relationship to blastomere symmetry or implantation potential, but increases with increased embryo fragmentation.
Rights and permissions
About this article
Cite this article
Combelles, C.M.H., Holick, E.A. & Racowsky, C. Release of superoxide dismutase-1 by day 3 embryos of varying quality and implantation potential. J Assist Reprod Genet 29, 305–311 (2012). https://doi.org/10.1007/s10815-012-9711-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-012-9711-4