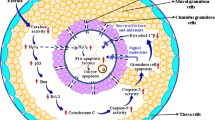
Abstract
Objective
Present study was aimed to determine whether aqueous neem leaf extract (NLE) induces generation of reactive oxygen species (ROS) and apoptosis through mitochondria-mediated pathway in rat oocytes.
Design
A controlled prospective study.
Setting
Laboratory research setting at Department of Zoology of Banaras Hindu University.
Animal(s)
Forty eight sexually immature female rats that were 20–30 days of age.
Intervention(s)
Sexually immature female rats were fed palatable dose of NLE (10 mg/g dry feed palate) for 10 days and then subjected to superovulation induction protocol. Thereafter, rats were euthanized, ovulated cumulus oocyte complexes were collected from oviduct and oocytes were denuded.
Main outcome measure(s)
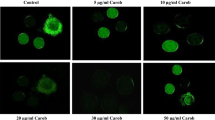
Rate of morphological apoptotic changes, measurement of hydrogen peroxide, nitric oxide and cytochrome c concentrations, caspase-9, caspases-3 activities and DNA fragmentation in oocytes.
Results
In vivo NLE treatment induced morphological apoptotic changes were associated with increased hydrogen peroxide, nitric oxide and cytochrome c concentrations, caspase-9, caspase-3 activities and DNA fragmentation in oocyte.
Conclusion
NLE induces generation of ROS that leads to oocytes apoptosis through mitochondria-mediated pathway.







Similar content being viewed by others
References
Subapriya R, Nagini S. Medicinal properties of neem leaves: a review. Curr Med Chem Anti-Canc Agents. 2005;5:149–56.
Chaube SK, Prasad PV, Khillare B, Shrivastav TG. Extract of Azadirachta indica (Neem) leaf induces apoptosis in rat oocytes cultured in vitro. Fertl Steril. 2006;85:1223–31.
Sithisarn P, Gritsanapan W. Variability of antioxidative quercetin content in Siamese neem tree leaves in Thailand by TLC-densitometry. Acta Hort (ISHS). 2008;786:161–9.
Lee YK, Hwang JT, Kwon DY, Surh YJ, Park OJ. Induction of apoptosis by quercetin is mediated through AMPKα1/ask1/p38 pathway. Cancer Lett. 2010;292:228–36.
Jeong JH, An JY, Kwon YT, Rhee JG, Lee YJ. Effects of low dose quercetin: cancer cell-specific inhibition of cell cycle progression. J Cell Biochem. 2009;106:73–82.
Kuo PL, Chen CY, Hsu YL. Isoobtusilactone A induces cell cycle arrest and apoptosis through reactive oxygen species/apoptosis signal-regulating kinase 1 signaling pathway in human breast cancer cells. Cancer Res. 2007;67:7406–20.
Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Update. 2004;7:97–110.
Cheng S, Gao N, Zhang Z, Chen G, Budhraja A, Ke Z, Son YO, Wang X, Luo J, Shi X. Quercetin induces tumor-selective apoptosis through downregulation of Mcl-1 and activation of bax. Clin Cancer Res. 2010;16:5679–91.
Chou CC, Yang JS, Lu HF, Ip SW, Lo C, Wu CC, Lin JP, Tang NY, Chung JG, Chou MJ, Teng YH, Chen DR. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Arch Pharm Res. 2010;33:1181–91.
Chien SY, Wu YC, Chung JG, Yang JS, Lu HF, Tsou MF, Wood WG, Kuo SJ, Chen DR. Quercetin-induced apoptosis acts through mitochondrial- and caspase-3-dependent pathways in human breast cancer MDA-MB-231 cells. Hum Exp Toxicol. 2010;28:493–503.
Lee YK, Park SY, Kim YM, Lee WS, Park OJ. AMP kinase/cyclooxygenase-2 pathway regulates proliferation and apoptosis of cancer cells treated with quercetin. Exp Mol Med. 2009;41:201–7.
Braun T, Dar S, Vorobiov D, Lindenboim L, Dascal N, Stein R. Expression of Bcl-x(S) in Xenopus oocytes induces BH3-dependent and caspase-dependent cytochrome c release and apoptosis. Mol Cancer Res. 2003;1:186–94.
Johnson CE, Freel CD, Kornbluth S. Features of programmed cell death in intact Xenopus oocytes and early embryos revealed by near-infrared fluorescence and real-time monitoring. Cell Death Differ. 2010;17:170–9.
Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–6.
Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–57.
Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–32.
Zhang X, Li XH, Ma X, Wang ZH, Lu S, Guo YL. Redox-induced apoptosis of human oocytes in resting follicles in vitro. J Soc Gynecol Investig. 2006;13:451–8.
Acehan D, Jiang X, Morgan DG, Heuser JE, Wang X, Akey CW. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol Cell. 2002;9:423–32.
Zou H, Li Y, Liu X, Wang X. An APAF-1 cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–56.
Fuentes-Prior P, Salvesen GS. The protein structures that shape caspase activity, specificity, activation and inhibition. Biochem J. 2004;384:201–32.
Martin MC, Allan LA, Lickrish M, Sampson C, Morrice N, Clarke PR. Protein kinase A regulates caspase-9 activation by Apaf-1 downstream of cytochrome c. J Biol Chem. 2005;280:15449–55.
Brown GC, Borutaite V. Regulation of apoptosis by the redox state of cytochrome c. Biochem Biophys Acta. 2008;1777:877–81.
Priyadarsini RV, Murugan RS, Maitreyi S, Ramalingam K, Karunagaran D, Nagini S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. Eur J Pharmacol. 2010;649:84–91.
Jung JH, Lee JO, Kim JH, Lee SK, You GY, Park SH, Park JM, Kim EK, Suh PG, An JK, Kim HS. Quercetin suppresses HeLa cell viability via AMPK-induced HSP70 and EGFR down-regulation. J Cell Physiol. 2010;223:408–14.
Chaube SK, Prasad PV, Thakur SC, Shrivastav TG. Hydrogen peroxide modulates meiotic cell cycle and induces morphological features characteristic of apoptosis in rat oocytes in vitro. Apoptosis. 2005;10:863–74.
Chaube SK, Dubey PK, Mishra SK, Prasad PV, Shrivastav TG. Calcium ionophore-induced egg activation or apoptosis is associated with the generation of intracellular hydrogen peroxide. Free Radic Res. 2008;42:212–20.
Chaube SK, Tripathi A, Khatun S, Mishra SK, Prasad PV, Shrivastav TG. Extracellular calcium protects against verapamil-induced metaphase-II arrest and initiation of apoptosis in aged rat eggs. Cell Biol Intl. 2009;33:337–43.
Tripathi A, Khatun S, Pandey AN, Mishra SK, Chaube R, Shrivastav TG, Chaube SK. Intracellular levels of hydrogen peroxide and nitric oxide in oocytes at various stages of meiotic cell cycle and apoptosis. Free Radic Res. 2009;43:287–94.
Harish Kumar G, Chandra Mohan KV, Jagannadha Rao A, Nagini S. Nimbolide a limonoid from Azadirachta indica inhibits proliferation and induces apoptosis of human choriocarcinoma (BeWo) cells. Invest New Drugs. 2009;27:246–52.
Harish Kumar G, Priyadarsini RV, Vinothini G, Vidjaya Letchoumy P, Nagini S. The neem limonoids azadirachtin and nimbolide inhibit cell proliferation and induce apoptosis in an animal model of oral oncogenesis. Invest New Drugs. 2010;28:392–401.
Jurisicova A, Acton BM. Deadly decisions: the role of genes regulating programmed cell death in human preimplantation embryo development. Reproduction. 2004;128:281–91.
Ansari B, Coates PJ, Greenstein BD, Hall PA. In situ end-labelling detects DNA strand breaks in apoptosis and other physiological and pathological states. J Pathol. 1993;170:1–8.
Hao Y, Lai L, Mao J, Im GS, Bonk A, Prather RS. Apoptosis in parthenogenetic preimplantation porcine embryos. Biol Reprod. 2004;70:1644–9.
Henkel R, Hajimohammad M, Stalf T, Hoogendijk C, Mehnert C, Menkveld R, Gips H, Schill WB, Kruger TF. Influence of deoxyribonucleic acid damage on fertilization and pregnancy. Fertl Steril. 2004;81:965–72.
Roth Z, Hansen PJ. Involvement of apoptosis in disruption of developmental competency of bovine oocytes by heat shock during maturation. Biol Reprod. 2004;71:1898–906.
Acknowledgement
Authors are thankful to Department of Science and Technology, New Delhi, India for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
Aqueous Neem (Azadirachta indica) leaf extract induces generation of ROS and thereby oocyte apoptosis through mitochondria-mediated pathway
Rights and permissions
About this article
Cite this article
Tripathi, A., Shrivastav, T.G. & Chaube, S.K. Aqueous extract of Azadirachta indica (neem) leaf induces generation of reactive oxygen species and mitochondria-mediated apoptosis in rat oocytes. J Assist Reprod Genet 29, 15–23 (2012). https://doi.org/10.1007/s10815-011-9671-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-011-9671-0