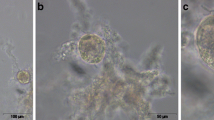
Abstract
Purpose
To assess the reliability of trypan blue (TB) and calcein AM/ethidium homodimer-1 (CaAM/EthD-1) staining to evaluate the viability of fresh and thawed human ovarian follicles.
Methods
Isolated follicles from fresh and thawed cortex were stained using TB versus CaAM/EthD-1 methods (n = 10 patients). Measurements were performed by two independent observers. The reliability was evaluated by intraclass correlation coefficient (ICC) and the differences between paired measurements were tested by the Wilcoxon test.
Results
Inter-observer reliability was excellent for each method. Nevertheless, it was even better with the TB method (ICC = 0.83) compared with CaAM/EthD-1 (ICC = 0.75). Moreover, the ICCs for viability measurements using the two methods were good for each observer (observer 1: ICC = 0.49; observer 2: ICC = 0.40).
Conclusion
Compared with CaAM/EthD-1, TB appears to be more reliable as a staining method for follicle viability evaluation. TB staining is a quick and useful method, complementary to histological analysis for quality control in ovarian tissue cryopreservation.



Similar content being viewed by others
References
Revel A, Revel-Vilk S. Fertility preservation in young cancer patients. J Hum Reprod Sci. 2010;3:2–7.
Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–10.
Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318–21.
Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin’s disease. Oncologist. 2007;12:1437–42.
Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, et al. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23:2266–72.
Silber SJ, DeRosa M, Pineda J, Lenahan K, Grenia D, Gorman K, et al. A series of monozygotic twins discordant for ovarian failure: ovary transplantation (cortical versus microvascular) and cryopreservation. Hum Reprod. 2008;23:1531–7.
Piver P, Amiot C, Agnani G, Pech J, Rohrlich PS, Vidal E, et al. Two pregnancies obtained after a new technique of autotransplantation of cryopreserved ovarian tissue. In: 25th Annual Meeting of ESHRE, 28 June – 1 July, 2009. Amsterdam, the Netherlands: Oxford University Press. Hum Reprod. 2009:i15.
Ernst E, Bergholdt S, Jørgensen JS, Andersen CY. The first woman to give birth to two children following transplantation of frozen/thawed ovarian tissue. Hum Reprod. 2010;25:1280–1.
Sánchez-Serrano M, Crespo J, Mirabet V, Cobo AC, Escribá MJ, Simón C, et al. Twins born after transplantation of ovarian cortical tissue and oocyte vitrification. Fertil Steril. 2010;93:268.e11–3.
Roux C, Amiot C, Agnani G, Aubard Y, Rohrlich PS, Piver P. Live birth after ovarian tissue autograft in a patient with sickle cell disease treated by allogeneic bone marrow transplantation. Fertil Steril. 2010;93:2413.e15–9.
Silber S, Kagawa N, Kuwayama M, Gosden R. Duration of fertility after fresh and frozen ovary transplantation. Fertil Steril. 2010;94:2191–6.
Revel A, Laufer N, Ben Meir A, Lebovich M, Mitrani E. Micro-organ ovarian transplantation enables pregnancy: a case report. Hum Reprod. 2011;26:1097–103.
Donnez J, Dolmans MM. Preservation of fertility in females with haematological malignancy. Br J Haematol. 2011;154:175–84.
Pegg DE, Jacobsen IA, Armitage WJ, Taylor MJ. Mechanisms of cryoinjury in organs. In: Pegg DE, Jacobsen IA, editors. Organ preservation, vol. 2. Edinbourgh: Churchill Livingstone; 1979. p. 132–46.
Nugent D, Newton H, Gosden RG, Rutherford AJ. Investigation of follicle survival after human heterotopic grafting. Hum Reprod. 1998;13:23–4.
Dolmans MM, Michaux N, Camboni A, Martinez-Madrid B, Van Langendonckt A, Nottola SA, et al. Evaluation of Liberase, a purified enzyme blend, for the isolation of human primordial and primary ovarian follicles. Hum Reprod. 2006;21:413–20.
Fauque P, Ben Amor A, Joanne C, Agnani G, Bresson JL, Roux C. Use of trypan blue staining to assess the quality of ovarian cryopreservation. Fertil Steril. 2007;87:1200–7.
Martinez-Madrid B, Dolmans MM, Langendonckt AV, Defrère S, Van Eyck AS, Donnez J. Ficoll density gradient method for recovery of isolated human ovarian primordial follicles. Fertil Steril. 2004;82:1648–53.
Maltaris T, Dragonas C, Hoffmann I, Mueller A, Beckmann MW, Dittrich R. Simple prediction of the survival of follicles in cryopreserved human ovarian tissue. J Reprod Dev. 2006;52:577–82.
Schubert B, Canis M, Darcha C, Artonne C, Pouly JL, Déchelotte P, et al. Human ovarian tissue from cortex surrounding benign cysts: a model to study ovarian tissue cryopreservation. Hum Reprod. 2005;20:1786–92.
Cortvrindt RG, Smitz JE. Fluorescent probes allow rapid and precise recording of follicle density and staging in human ovarian cortical biopsy samples. Fertil Steril. 2001;75:588–93.
Sheskin DJ. Handbook of parametric and nonparametric statistical procedures. 2nd ed. Chapmann & Hall ⁄ CRC, 2000.
Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–8.
Smith CAB. On the estimation of intraclasscorrelation. Ann Hum Genet. 1956;21:363–73.
Bartko JJ. The intraclass correlation coefficient as a measure of reliability. Psychol Rep. 1966;19:3–11.
Shourki MM, Pause CA. Statistical methods for health sciences. 2nd ed. CRC Press LLC, 1999.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10.
Bland JM, Altman DG. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet. 1995;346:1085–7.
Schmidt KL, Andersen CY, Loft A, Byskov AG, Ernst E, Andersen AN. Follow-up of ovarian function post-chemotherapy following ovarian cryopreservation and transplantation. Hum Reprod. 2005;20:3539–46.
Schubert B, Canis M, Darcha C, Artonne C, Smitz J, Grizard G. Follicular growth and estradiol follow-up after subcutaneous xenografting of fresh and cryopreserved human ovarian tissue. Fertil Steril. 2008;89:1787–94.
Oktay K, Nugent D, Newton H, Salha O, Chatterjee P, Gosden RG. Isolation and characterization of primordial follicles from fresh and cryopreserved human ovarian tissue. Fertil Steril. 1997;67:481–6.
Vanacker J, Camboni A, Dath C, Van Langendonckt A, Dolmans MM, Donnez J, Amorim CA. Enzymatic isolation of human primordial and primary ovarian follicles with Liberase DH: protocol for application in a clinical setting. Fertil Steril 2011 Jun 28. [Epub ahead of print]
Poeschmann M, Fassbender M, Lopes C, Dorresteijn A, Jewgenow K. Viability assessment of primordial follicles of domestic cats to develop cryopreservation protocols for ovarian tissue. Reprod Domest Anim. 2008;43 Suppl 1:25.
Lopes CA, dos Santos RR, Celestino JJ, Melo MA, Chaves RN, Campello CC, et al. Short-term preservation of canine preantral follicles: effects of temperature, medium and time. Anim Reprod Sci. 2009;115:201–14.
Merdassi G, Mazoyer C, Guerin JF, Saad A, Salle B, Lornage J. Examination of viability and quality of ovarian tissue after cryopreservation using simple laboratory methods in ewe. Reprod Biol Endocrinol. 2011;9:78.
Acknowledgements
We would like to thank all the surgical team in the department of gynecology, CHU Estaing, Clermont-Ferrand (France) for providing ovarian tissue for research, Dr Benoît Sion for his critical recommendations on this study and Mrs Elizabeth Petit for language revision of the manuscript. Thanks are also due to Mr Eric Pernet (CICE) for advice and technical assistance.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule Trypan blue appears to be a more reliable staining method for follicle viability evaluation compared with calcein AM/ethidium homodimer-1
Supported by: an industrial doctoral grant (Convention Industrielle de Formation par la Recherche, CIFRE) with the Centre International de Chirurgie Endoscopique (CICE), France (Grant No: 176/2009).
Rights and permissions
About this article
Cite this article
Sanfilippo, S., Canis, M., Ouchchane, L. et al. Viability assessment of fresh and frozen/thawed isolated human follicles: reliability of two methods (Trypan blue and Calcein AM/ethidium homodimer-1). J Assist Reprod Genet 28, 1151–1156 (2011). https://doi.org/10.1007/s10815-011-9649-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-011-9649-y