Abstract
Purpose
To investigate the effects of cumulus cells removal after 6 h co-incubation of gametes on the fertilization, polyspermy, multinucleation and clinical pregnancy rates in human IVF.
Methods
A total of 1,200 IVF-ET cycles undergoing 6 h co-incubation of gametes in 2009 were included in this study. Inclusion criteria were: female age <38 years, first IVF treatment, with bi-ovary and normal ovarian response, e.g., 4 ~ 20 oocytes could be obtained. A 6 h period of co-incubation was applied in all IVF cycles. According to the history of infertility, cumulus cells were mechanically removed either 6 h post-insemination or 20 h post-insemination. For couples with primary infertility, or unexplained infertility, or mild oligospermia or asthenospermia, the cumulus cells were removed at 6 h of insemination for the polar body observation (6 h group, n = 565). Of these, 80 cycles received early rescue ICSI due to fertilization failure or low fertilization rate at 6 h of insemination. For couples with secondary infertility and normal semen analysis, the cumulus cells were removed at 20 h of insemination as routine (20 h group, n = 635). Of these, three cycles received late rescue ICSI due to fertilization failure at 20 h of insemination. Normal fertilization, polyspermy (≥3PN), multinucleation and clinical pregnancy rates were compared between the two groups (rescue ICSI cycles were not included in the comparison in both groups).
Results
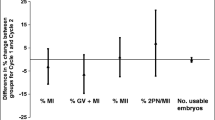
Significant difference (P < 0.05) was observed between the groups regarding polyspermy rates (7.48% in 6 h group and 9.22% in 20 h group). No difference was observed between the groups regarding normal fertilization rates (2PN rate) (64.89% in 6 h group and 65.74% in 20 h group). No difference was observed between the groups regarding multinucleation and clinical pregnancy rates (11.01% and 65.15% in 6 h group, 10.75% and 66.93% in 20 h group, respectively). The clinical pregnancy rate was 51.43% in cycles receiving early rescue ICSI, while no clinical pregnancy was obtained in cycles receiving late rescue ICSI.
Conclusion
The present results indicate that cumulus cells removal at 6 h of insemination is a relatively safe operation, which yielded comparable normal fertilization rate, multinucleation and clinical pregnancy rates compared with 20 h group. This protocol may be beneficial for early obsevation of fertilization failure and make early rescue ICSI possible.
Similar content being viewed by others
References
Aitken RJ. A free radical theory of male infertility. Reprod Fertil Dev. 1994;6:19–24.
Aitken RJ. Pathophysiology of human spermatozoa. Curr Opin Obstel Gynecol. 1994;6:128–35.
Bungum M, Bungum L, Humaidan P. A prospective study, using sibling oocytes, examining the effect of 30 seconds versus 90 min gamete co-incubation in IVF. Hum Reprod. 2006;21:518–23.
Gianaroli L, Fiorentino A, Cristina Magli M, Ferraretti AP, Montanaro M. Prolonged sperm-oocyte exposure and high sperm concentration affect human embryo viability and pregnancy rate. Hum Reprod. 1996;11:2507–11.
Dirnfeld M, Bider D, Koifman M, Calderon I, Abramovici H. Shortened exposure of oocytes to spermatozoa improves in-vitro fertilization outcome: a prospective, randomized, controlled study. Hum Reprod. 1999;14:2562–4.
Kattera S, Chen C. Short coincubation of gametes in in vitro fertilization improves implantation and pregnancy rates: a prospective, randomized, controlled study. Fertil Steril. 2003;80:1017–21.
Nagy ZP, Joris H, Liu J, Staessen C, Devroey P, Van Steirteghem AC. Intracytoplasmic single sperm injection of 1-day-old unfertilized human oocytes. Hum Reprod. 1993;8:2180–4.
Morton PC, Yoder CS, Tucker MJ, Wright G, Brockman WD, Kort HI. Reinsemination by intracytoplasmic sperm injection of 1-day-old oocytes after complete conventional fertilization failure. Fertil Steril. 1997;68:488–91.
Chen C, Kattera S. Rescue ICSI of oocytes that failed to extrude the second polar body 6 h post-insemination in conventional IVF. Hum Reprod. 2003;18:2118–21.
Lundqvist M, Johansson U, Lundkvist O, Milton K, Westin C, Simberg N. Reducing the time of co-incubation of gametes in human in-vitro fertilization has no beneficial effects. Reprod Biomed Online. 2001;3:21–4.
Tao T, Robichaud A, Nadeau S, Savoie R, Gallant B, Ouellette R. Effect of cumulus cell removal on the fertilization and the day 3 embryo quality in human IVF. Int Congr Ser. 2004;1271:135–8.
Ye H, Huang G-N, Zeng P-H, Pei L. IVF/ICSI outcomes between cycles with luteal estradiol (E2) pre-treatment before GnRH antagonist protocol and standard long GnRH agonist protocol: a prospective and randomized study. J Assist Reprod Genet. 2009;26:105–11.
Dale B, Elder K. In vitro Fertilization. United Kingdom: Cambrige University; 1997. p. 115–6.
Chen C, Sathananthan H. Early penetration of human sperm through the vestments of human eggs in vitro. Arch Androl. 1986;16:183–97.
Plachot M, Junca AM, Mandelbaum J, Cohen J, Salat-Baroux J, Da Lage C. Timing of in-vitro fertilization of cumulus free and cumulus enclosed human oocytes. Hum. Reprod 1986:1237–1242.
Payne D, Flaherty SP, Barry MF, Matthews CD. Preliminary observation on polar body extrusion and pronuclear formation in human oocytes using time-lapse video cinematography. Hum Reprod. 1997;12:532–41.
Nagy ZP, Liu J, Joris H, Devroey P, Van Steirteghem A. Time-course of oocyte activation, pronucleus formation and cleavage in human oocytes fertilized by intracytoplasmic sperm injection. Hum Reprod. 1994;9:1743–8.
Van Soom A, Tanghe S, De Pauw I, Maes D, de Kruif A. Function of the Cumulus Oophorus Before and During Mammalian Fertilization. Reprod Dom Anim. 2002;37:144–51.
Gianaroli L, Cristina Magli M, Pia Ferraretti A, Fiorentino A, Tosti E, Panzella S, et al. Reducing the time of sperm-oocyte interaction in human in-vitro fertilization improves the implantation rate. Hum Reprod. 1996;11:166–71.
Pieters MH, Dumoulin JC, Ignoul-Vanvuchelen RC, Bras M, Evers JL, Geraedts JP. Triploidy after in vitro fertilization: cytogenetic analysis of human zygotes and embryos. J Assist Reprod Genet. 1992;9:68–76.
Tucker MJ, Bishop FM, Cohen J, Wiker SR, Wright G. Routine application of partial zona dissection for male factor infertility. Hum Reprod. 1991;6:676–81.
Matt DW, Ingram AR, Graff DP, Edelstein MC. Normal birth after single-embryo transfer in a patient with excessive polypronuclear zygote formation following in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. 2004;6:1662–5.
Sathananthan AH, Trounson AO. Ultrastructure of cortical granule release and zona interaction in monospermic and polyspermic human ova fertilized in vitro. Gamete Research. 1982;6:225–34.
van der Ven HH, Al-Hasani S, Diedrich K, Hamerich U, Lehmann F, Krebs D. Polyspermy in in-vitro fertilization of human oocytes: frequency and possible causes. Ann N Y Acad Sci. 1985;442:88–95.
Van Royen E, Mangelschots K, Vercruyssen M, De Neubourg D, Valkenburg M, Ryckaert G, et al. Multinucleation in cleavage stage embryos. Hum Reprod. 2003;18:1062–9.
Chen HL, Copperman AB, Grunfeld L, Sandler B, Bustillo M, Gordon JW. Failed fertilization in vitro: Second day micromanipulation of oocytes versus reinsemination. Fertil Steril. 1995;63:1337–40.
Nagy ZP, Rienzi LF, Ubaldi FM, Greco E, Massey JB, Kort HI. Effect of reduced oocyte aging on the outcome of rescue intracytoplasmic sperm injection. Fertil Steril. 2006;85:901–6.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule Removal of cumulus 6 hours after the initiation of IVF is practical, safe, and offers advantages when early rescue ICSI is indicated.
Rights and permissions
About this article
Cite this article
Xiong, S., Han, W., Liu, J.X. et al. Effects of cumulus cells removal after 6 h co-incubation of gametes on the outcomes of human IVF. J Assist Reprod Genet 28, 1205–1211 (2011). https://doi.org/10.1007/s10815-011-9630-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-011-9630-9