Abstract
Purpose
To understand the molecular basis of sperm-motility and to identify related novel motility biomarkers.
Methods
Two-dimensional electrophoresis (2DE) followed by Reverse-phase-nano-high-performance liquid chromatography-electrospray ionization tandem mass spectrometry (RP-nano-HPLC-ESI-MS/MS) were applied to establish the human sperm proteome. Then the sperm proteome of moderate-motile human sperm fraction and that of good-motile human sperm fraction from pooled spermatozoa of forty normozoospermic donors (Group 1 subjects) were compared to identify the dysregulated proteins. Among these down-regulated proteins, Protein tyrosine phosphatase non-receptor type 14 (PTPN14) was chosen to reconfirm by Western blotting and semi-quantitative reverse transcription polymerase chain reaction. For clinical application, Western blotting and real-time reverse transcription polymerase chain reaction was performed to compare the expression level of PTPN14 in (Group 2 subjects) nine normozoospermic controls and thirty-three asthenozoospermic patients (including 21 mild asthenozoospermic cases and 12 severe cases). Finally, bioinformatic tools prediction and immunofluorescence assay were performed to elucidate the potential localization of PTPN14.
Results
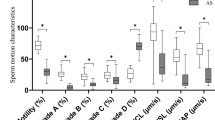
The expression levels of three proteins were observed to be lower in the moderate-motile sperm fraction than in good-motile sperm of group 1 subjects. Among three proteins with persistent down-regulation in the moderate-motile sperm, we reconfirmed that the expression level of PTPN14 was significantly lower in both mRNA and protein levels from the moderate-motile sperm fraction. Further, down-regulation of PTPN14 was found at the translational and transcriptional level in the asthenozoospermic men. Finally, Bioinformatic tools prediction and immunofluorescence assay showed that PTPN14 maybe predominantly localized at the mitochondria in the midpiece of human ejaculated sperm.
Conclusions
Proteomics tools were applied to identify three possible sperm motility-related proteins. Among these proteins, PTPN14 was highly likely a novel sperm-motility biomarker and a potential mitochondrial protein.





Similar content being viewed by others
References
Greenhall E, Vessey M. The prevalence of subfertility: a review of the current confusion and a report of two new studies. Fertil Steril. 1990;54:978–83.
Boyle CA, Khoury MJ, Katz DF, Annest JL, Kresnow MJ, DeStefano F, et al. The relation of computer-based measures of sperm morphology and motility to male infertility. Epidemiology. 1992;3:239–46.
World Health Organization. WHO, Laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed. Cambridge: Cambridge University Press; 1999.
Curi SM, Ariagno JI, Chenlo PH, Mendeluk GR, Pugliese MN, Sardi Segovia LM, et al. Asthenozoospermia: analysis of a large population. Arch Androl. 2003;49:343–9.
Leclerc P, de Lamirande E, Gagnon C. Interaction between Ca2+, cyclic 3′,5′ adenosine monophosphate, the superoxide anion, and tyrosine phosphorylation pathways in the regulation of human sperm capacitation. J Androl. 1998;19:434–43.
Leclerc P, de Lamirande E, Gagnon C. Regulation of protein-tyrosine phosphorylation and human sperm capacitation by reactive oxygen derivatives. Free Radic Biol Med. 1997;22:643–56.
Leclerc P, de Lamirande E, Gagnon C. Cyclic adenosine 3′,5′monophosphate-dependent regulation of protein tyrosine phosphorylation in relation to human sperm capacitation and motility. Biol Reprod. 1996;55:684–92.
Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA, Pan D, et al. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development. 1995;121:1139–50.
Lin M, Lee YH, Xu W, Baker MA, Aitken RJ. Ontogeny of tyrosine phosphorylation-signaling pathways during spermatogenesis and epididymal maturation in the mouse. Biol Reprod. 2006;75:588–97.
de Lamirande E, Gagnon C. Human sperm hyperactivation and capacitation as parts of an oxidative process. Free Radic Biol Med. 1993;14:157–66.
Aitken RJ, Paterson M, Fisher H, Buckingham DW, van Duin M. Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J Cell Sci. 1995;108(Pt 5):2017–25.
de Lamirande E, Gagnon C. Capacitation-associated production of superoxide anion by human spermatozoa. Free Radic Biol Med. 1995;18:487–95.
Aitken RJ, Harkiss D, Knox W, Paterson M, Irvine DS. A novel signal transduction cascade in capacitating human spermatozoa characterised by a redox-regulated, cAMP-mediated induction of tyrosine phosphorylation. J Cell Sci. 1998;111(Pt 5):645–56.
Baker MA, Hetherington L, Aitken RJ. Identification of SRC as a key PKA-stimulated tyrosine kinase involved in the capacitation-associated hyperactivation of murine spermatozoa. J Cell Sci. 2006;119:3182–92.
Lewis B, Aitken RJ. A redox-regulated tyrosine phosphorylation cascade in rat spermatozoa. J Androl. 2001;22:611–22.
Hecht D, Zick Y. Selective inhibition of protein tyrosine phosphatase activities by H2O2 and vanadate in vitro. Biochem Biophys Res Commun. 1992;188:773–9.
Nolan MA, Babcock DF, Wennemuth G, Brown W, Burton KA, McKnight GS. Sperm-specific protein kinase A catalytic subunit Calpha2 orchestrates cAMP signaling for male fertility. Proc Natl Acad Sci USA. 2004;101:13483–8.
Holt WV, Harrison RA. Bicarbonate stimulation of boar sperm motility via a protein kinase A-dependent pathway: between-cell and between-ejaculate differences are not due to deficiencies in protein kinase A activation. J Androl. 2002;23:557–65.
Lawson C, Goupil S, Leclerc P. Increased activity of the human sperm tyrosine kinase SRC by the cAMP-dependent pathway in the presence of calcium. Biol Reprod. 2008;79:657–66.
Martinez-Heredia J, Estanyol JM, Ballesca JL, Oliva R. Proteomic identification of human sperm proteins. Proteomics. 2006;6:4356–69.
de Mateo S, Martinez-Heredia J, Estanyol JM, Dominguez-Fandos D, Vidal-Taboada JM, Ballesca JL, et al. Marked correlations in protein expression identified by proteomic analysis of human spermatozoa. Proteomics. 2007;7:4264–77.
Zhao C, Huo R, Wang FQ, Lin M, Zhou ZM, Sha JH. Identification of several proteins involved in regulation of sperm motility by proteomic analysis. Fertil Steril. 2007;87:436–8.
Martinez-Heredia J, de Mateo S, Vidal-Taboada JM, Ballesca JL, Oliva R. Identification of proteomic differences in asthenozoospermic sperm samples. Hum Reprod. 2008;23:783–91.
Siva AB, Kameshwari DB, Singh V, Pavani K, Sundaram CS, Rangaraj N, et al. Proteomics-based study on asthenozoospermia: differential expression of proteasome alpha complex. Mol Hum Reprod. 2010;16:452–62.
Chan CC, Shui HA, Wu CH, Wang CY, Sun GH, Chen HM, et al. Motility and protein phosphorylation in healthy and asthenozoospermic sperm. J Proteome Res. 2009;8:5382–6.
White DR, Phillips DM, Bedford JM. Factors affecting the acrosome reaction in human spermatozoa. J Reprod Fertil. 1990;90:71–80.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.
Shevchenko A, Jensen ON, Podtelejnikov AV, Sagliocco F, Wilm M, Vorm O, et al. Linking genome and proteome by mass spectrometry: large-scale identification of yeast proteins from two dimensional gels. Proc Natl Acad Sci USA. 1996;93:14440–5.
Tyan YC, Wu HY, Su WC, Chen PW, Liao PC. Proteomic analysis of human pleural effusion. Proteomics. 2005;5:1062–74.
Bauer MF, Hofmann S, Neupert W, Brunner M. Protein translocation into mitochondria: the role of TIM complexes. Trends Cell Biol. 2000;10:25–31.
Ficarro S, Chertihin O, Westbrook VA, White F, Jayes F, Kalab P, et al. Phosphoproteome analysis of capacitated human sperm. Evidence of tyrosine phosphorylation of a kinase-anchoring protein 3 and valosin-containing protein/p97 during capacitation. J Biol Chem. 2003;278:11579–89.
Peddinti D, Nanduri B, Kaya A, Feugang JM, Burgess SC, Memili E. Comprehensive proteomic analysis of bovine spermatozoa of varying fertility rates and identification of biomarkers associated with fertility. BMC Syst Biol. 2008;2:19.
Gonzalez-Fernandez L, Ortega-Ferrusola C, Macias-Garcia B, Salido GM, Pena FJ, Tapia JA. Identification of protein tyrosine phosphatases and dual-specificity phosphatases in mammalian spermatozoa and their role in sperm motility and protein tyrosine phosphorylation. Biol Reprod. 2009;80:1239–52.
Carra E, Sangiorgi D, Gattuccio F, Rinaldi AM. Male infertility and mitochondrial DNA. Biochem Biophys Res Commun. 2004;322:333–9.
McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16:R551–60.
Guo X, Zhao C, Wang F, Zhu Y, Cui Y, Zhou Z, et al. Investigation of human testis protein heterogeneity using 2-dimensional electrophoresis. J Androl. 2010;31:419–29.
Bohring C, Krause E, Habermann B, Krause W. Isolation and identification of sperm membrane antigens recognized by antisperm antibodies, and their possible role in immunological infertility disease. Mol Hum Reprod. 2001;7:113–8.
Bohring C, Krause W. The characterization of human spermatozoa membrane proteins–surface antigens and immunological infertility. Electrophoresis. 1999;20:971–6.
Alonso A, Sasin J, Bottini N, Friedberg I, Osterman A, Godzik A, et al. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711.
Edwards K, Davis T, Marcey D, Kurihara J, Yamamoto D. Comparative analysis of the Band 4.1/ezrin-related protein tyrosine phosphatase Pez from two Drosophila species: implications for structure and function. Gene. 2001;275:195–205.
Ogata M, Takada T, Mori Y, Uchida Y, Miki T, Okuyama A, et al. Regulation of phosphorylation level and distribution of PTP36, a putative protein tyrosine phosphatase, by cell-substrate adhesion. J Biol Chem. 1999;274:20717–24.
Tsukita S, Yonemura S, Tsukita S. ERM proteins: head-to-tail regulation of actin-plasma membrane interaction. Trends Biochem Sci. 1997;22:53–8.
Ogata M, Takada T, Mori Y, Oh-hora M, Uchida Y, Kosugi A, et al. Effects of overexpression of PTP36, a putative protein tyrosine phosphatase, on cell adhesion, cell growth, and cytoskeletons in HeLa cells. J Biol Chem. 1999;274:12905–9.
Barr AJ, Debreczeni JE, Eswaran J, Knapp S. Crystal structure of human protein tyrosine phosphatase 14 (PTPN14) at 1.65-A resolution. Proteins. 2006;63:1132–6.
Wadham C, Gamble JR, Vadas MA, Khew-Goodall Y. Translocation of protein tyrosine phosphatase Pez/PTPD2/PTP36 to the nucleus is associated with induction of cell proliferation. J Cell Sci. 2000;113(Pt 17):3117–23.
Tash JS, Bracho GE. Regulation of sperm motility: emerging evidence for a major role for protein phosphatases. J Androl. 1994;15:505–9.
Han Y, Haines CJ, Feng HL. Role(s) of the serine/threonine protein phosphatase 1 on mammalian sperm motility. Arch Androl. 2007;53:169–77.
Devi KU, Jha K, Shivaji S. Plasma membrane-associated protein tyrosine phosphatase activity in hamster spermatozoa. Mol Reprod Dev. 1999;53:42–50.
Tomes CN, Roggero CM, De Blas G, Saling PM, Mayorga LS. Requirement of protein tyrosine kinase and phosphatase activities for human sperm exocytosis. Dev Biol. 2004;265:399–415.
Seligman J, Zipser Y, Kosower NS. Tyrosine phosphorylation, thiol status, and protein tyrosine phosphatase in rat epididymal spermatozoa. Biol Reprod. 2004;71:1009–15.
Swarbrick MM, Havel PJ, Levin AA, Bremer AA, Stanhope KL, Butler M, et al. Inhibition of protein tyrosine phosphatase-1B with antisense oligonucleotides improves insulin sensitivity and increases adiponectin concentrations in monkeys. Endocrinology. 2009;150:1670–9.
Acknowledgement
This study was supported by grants from the National Science Council of the Republic of China (NSC- 91-2314-B-006-149, NSC 91-3112-B-006-008, NSC 92-3112-B-006-002, NSC 93-3112-B-006-004, and NSC 93-2314-B-006-078).
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
Protein tyrosine phosphatase non-receptor type 14 is a novel sperm-motility biomarker proven by proteomic tools.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Table 1
Parameters for good-motility and moderate-motility sperm fractions which were separated by density gradients and used for proteomics study. (DOC 29 kb)
Supplemental Table 2
Sperm parameters of normozoospermic controls and men with asthenozoospermia. (DOC 30 kb)
Supplemental Table 3
Proteins which are persistently under-expressed in moderate-motility sperm. (DOC 29 kb)
Rights and permissions
About this article
Cite this article
Chao, HC.A., Chung, CL., Pan, HA. et al. Protein tyrosine phosphatase non-receptor type 14 is a novel sperm-motility biomarker. J Assist Reprod Genet 28, 851–861 (2011). https://doi.org/10.1007/s10815-011-9602-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-011-9602-0