Abstract
Purpose
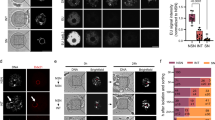
The final stages of antral mouse oocytes maturation are characterised by the transition from transcriptionally active NSN to inactive SN oocytes. Here, we studied the profile of histone acetylation changes occurring during the NSN-to-SN transition.
Methods
During the NSN-to-SN transition, oocytes were classified based on their chromatin organisation and the immunocytochemical profile of histones H2B, H3 and H4 acetylation was analysed.
Results
We described four patterns of immunostaining common to the three acetylated histones, corresponding to stages of progressive localisation: From a diffused distribution in NSN oocytes to the association with constitutive heterochromatin and then to the nucleolar surface region in SN oocytes.
Conclusions
The maintenance of a favourable transcriptional epigenetic context, in the heterochromatin of transcriptionally silent SN oocytes, might be related to the early stages of development when transcripts from these heterochromatic regions are functional to preimplantation progression.

Similar content being viewed by others
References
Bouniol-Baly C, Hamraoui L, Guibert J, Beaujean N, Szollosi MS, Debey P. Differential transcriptional activity associated to chromatin configuration in fully grown germinal vesicle mouse oocytes. Biol Reprod. 1999;60:580–7.
Bui HT, Van Thuan N, Kishigami S, Wakayama S, Hikichi T, Ohta H, et al. Regulation of chromatin and chromosome morphology by histone H3 modifications in pig oocytes. Reproduction. 2007;133:371–82.
Chambeyron S, Bickmore WA. Does looping and clustering in the nucleus regulate gene expression? Curr Opin Cell Biol. 2004;16:256–62.
Christians E, Boiani M, Garagna S, Dessy C, Redi CA, Renard JP, et al. Gene expression and chromatin organization during mouse oocyte growth. Dev Biol. 1999;207:76–85.
Debey P, Szollozi MS, Szollosi D, Vautier D, Girouse A, Besombes D. Competent mouse oocytes isolated from antral follicles exhibit different chromatin organization and follow different maturation dynamics. Molec Reprod Dev. 1993;36:59–74.
De La Fuente R. Chromatin modifications in the germinal vesicle (GV) of mammalian oocytes. Dev Biol. 2006;292:1–12.
De La Fuente R, Viveiros MM, Burns KH, Adashi EY, Matzuk MM, Eppig JJ. Major chromatin remodeling in the germinal vesicle (GV) of mammalian oocytes is dispensable for global transcriptional silencing but required for centromeric heterochromatin function. Dev Biol. 2004;275:447–58.
Foygel K, Choi B, Jun S, Leong DE, Lee A, Wong CC, et al. A novel and critical role for Oct4 as a regulator of the maternal-embryonic transition. PLoS One. 2008;3(12):e4109.
Inoue A, Nakajima R, Nagata M, Aoki F. Contribution of the oocyte nucleus and cytoplasm to the determination of meiotic and developmental competence in mice. Human Reprod. 2008;23:1377–84.
Kageyama S, Liu H, Kaneko N, Ooga M, Nagata M, Aoki F. Alterations in epigenetic modifications during oocyte growth in mice. Reproduction. 2007;133:85–94.
Kaplan G, Abreu S, Bachvarova R. rRNA accumulation and protein synthetic patterns in growing mouse oocytes. J Exp Zool. 1982;220:361–70.
Kim JM, Liu H, Tazaki M, Nagata M, Aoki F. Changes in histone acetylation during mouse oocyte meiosis. J Cell Biol. 2003;162:37–46.
Li L, Zheng P, Dean J. Maternal control of early mouse development. Development. 2010;137:859–70.
Liu H, Aoki F. Transcription activity associated with meiotic competence in fully grown mouse GV oocytes. Zygote. 2002;10:327–32.
Longo F, Garagna S, Merico V, Orlandini G, Gatti R, Scandroglio R, et al. Nuclear localization of NORs and centromeres in mouse oocytes during folliculogenesis. Mol Reprod Dev. 2003;66:279–90.
Mattson DA, Albertini DF. Oogenesis: chromatin and microtubule dynamics during meiotic prophase. Mol Reprod Dev. 1990;25:374–83.
Miyara F, Migne C, Dumont-Hassan M, Le Meur A, Cohen-Bacrie P, Aubriot F, et al. Chromatin configuration and transcriptional control in human and mouse oocytes. Mol Reprod Dev. 2003;64:458–70.
Moore GPM, Lintern-Moore S, Peters H, Faber M. RNA synthesis in the mouse oocyte. J Cell Biol. 1974;60:416–22.
Ola SI, Wang Q, Ai JS, Yin S, Liang CG, Chen DY, et al. Meiotic competence and acetylation pattern of UV light classified mouse antral oocytes after meiotic arrest with isobutylmethylxanthine. Mol Reprod Dev. 2007;74:591–9.
Payer B, Saitou M, Barton SC, Thresher R, Dixon JPC, Zahn D, et al. Stella is a maternal effect gene required for normal early development in mice. Current Biol. 2003;13:2110–7.
Probst AV, Okamoto I, Casanova M, El Marjou F, Le Baccon P, Almouzni G. A strand-specific burst in transcription of pericentric satellites is required for chromocenter formation and early mouse development. Dev Cell. 2010;19:625–38.
Shi L, Wu J. Epigenetic regulation in mammalian preimplantation embryo development. Reprod Biol Endocrinol. 2009;7:59–70.
Tan J, Wang H, Sun X, Liu Y, Sui H, Zhang J. Chromatin configurations in the germinal vesicle of mammalian oocytes. Mol Hum Reprod. 2009;15:1–9.
Zuccotti M, Piccinelli A, Giorgi Rossi P, Garagna S, Redi CA. Chromatin organization during mouse oocyte growth. Mol Reprod Dev. 1995;41:479–85.
Zuccotti M, Ponce RH, Boiani M, Guizzardi S, Govoni P, Scandroglio R, et al. The analysis of chromatin organization allows selection of mouse antral oocytes competent for development to blastocyst. Zygote. 2002;10:73–8.
Zuccotti M, Garagna S, Merico V, Monti M, Redi CA. Chromatin organisation and nuclear architecture in growing mouse oocytes. Mol Cell Endocrinol. 2005;234:11–7.
Zuccotti M, Merico V, Sacchi L, Bellone M, Brink TC, Bellazzi R, et al. Maternal Oct-4 is a potential key regulator of the developmental competence of mouse oocytes. BMC Dev Biol. 2008;8:97.
Zuccotti M, Merico V, Sacchi L, Bellone M, Brink TC, Stefanelli M, et al. Oct-4 regulates the expression of Stella and Foxj2 at the Nanog locus: implications for the developmental competence of mouse oocytes. Human Reprod. 2009;24:2225–37.
Zuccotti M, Merico V, Redi CA, Garagna S (2011) What does it take to make a mammalian developmentally competent oocyte? Human Reprod. Update; in press
Acknowledgements
We thank the following organisations for supporting this research: University of Parma (FIL), UNIPV-Regione Lombardia, Fondazione Alma Mater Ticinensis, Fondazione I.R.C.C.S. Policlinico San Matteo , and ‘Bando Giovani Ricercatori 2007’ to C.A.R.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Capsule In late antral mouse oocytes, chromatin condenses around the nucleolus, becomes transcriptionally silent, but maintains transcriptionally permissive histone acetylation profiles, likely functional to development.
Rights and permissions
About this article
Cite this article
Zuccotti, M., Bellone, M., Longo, F. et al. Fully-mature antral mouse oocytes are transcriptionally silent but their heterochromatin maintains a transcriptional permissive histone acetylation profile. J Assist Reprod Genet 28, 1193–1196 (2011). https://doi.org/10.1007/s10815-011-9562-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-011-9562-4