Abstract
Purpose
To evaluate possible differences in follicle and oocyte developmental competence after gonadotrophin treatment in sows of obese and lean genotypes.
Methods
Follicle dynamics, ovulation rate and oocyte developmental competence to embryo were compared between females, of obese (n = 7) and lean genotypes (n = 10), treated with 1,250 I.U. of eCG and 500 I.U. of hCG.
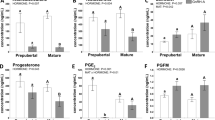
Results
The obese genotype showed lower numbers of follicles growing to preovulatory stages (12.4 ± 1.8 vs 18.6 ± 1.0, P < 0.05), of corpora lutea (16.0 ± 0.9 vs 23.5 ± 0.9, P < 0.05), and of recovered oocytes/embryos (8.0 ± 1.3 vs 12.9 ± 0.9, P < 0.05). Thereafter, embryo viability rates also decreased when compared to lean genotypes (62.5 vs 77.6%, P < 0.05).
Discussion
To our knowledge, this is the first study analyzing the effect of obese genotypes on the ovarian response to exogenous gonadotrophins in a non-rodent animal model, the pig. A lower efficiency of gonadotrophin treatments for stimulation of follicle development and induction of ovulation was observed.


Similar content being viewed by others
References
Nieto R, Miranda A, García MA, Aguilera JF. The effect of dietary protein content and feeding level on the rate of protein deposition and energy utilization in growing Iberian pigs from 15 to 50 kg body weight. Br J Nutr. 2002;88:39–49.
Fernandez-Figares I, Lachica M, Nieto R, Rivera-Ferre MG, Aguilera JF. Serum profile of metabolites and hormones in obese (Iberian) and lean (Landrace) growing gilts fed balanced or lysine deficient diets. Livestock Sci. 2007;110:73–81.
Brüssow KP, Schneider F, Tuchscherer A, Egerszegi I, Rátky J. Comparison of luteinizing hormone, leptin and progesterone levels in the systemic circulation (Vena jugularis) and near the ovarian circulation (Vena cava caudalis) during the oestrous cycle in Mangalica and Landrace gilts. J Reprod Develop. 2008;54:431–8.
Ovilo C, Fernandez A, Fernandez AI, Folch JM, Varona L, Benıtez R, Nunez Y, Rodrıguez C, Silio L. Hypothalamic expression of porcine leptin receptor (LEPR), neuropeptide Y (NPY), and cocaine- and amphetamine-regulated transcript (CART) genes is influenced by LEPR genotype. Mamm Genome. 2010 (in press, doi: 10.1007/s00335-010-9307-1).
Ovilo C, Fernández A, Noguera JL, Barragán C, Letón R, Rodríguez C, et al. Fine mapping of porcine chromosome 6 QTL and LEPR effects on body composition in multiple generations of an Iberian by Landrace intercross. Genet Res. 2005;85:57–67.
Muñoz G, Ovilo C, Silió L, Tomás A, Noguera JL, Rodríguez MC. Single- and joint-population analyses of two experimental pig crosses to confirm quantitative trait loci on Sus scrofa chromosome 6 and leptin receptor effects on fatness and growth traits. J Anim Sci. 2009;87:459–68.
Morales J, Pérez JF, Baucells MD, Mourot J, Gasa J. Comparative digestibility and lipogenic activity in Landrace and Iberian finishing pigs fed ad libitum corn- and corn–sorghum–acorn-based diets. Livest Prod Sci. 2002;77:195–205.
Myers MG, Cowley MA, Münzberg H. Mechanisms of leptin action and leptin resistance. Ann Rev Physiol. 2008;70:537–56.
Hoch M, Eberle AN, Wagner U, Bussmann C, Peters T, Peterli R. Expression and localization of melanocortin-1 receptor in human adipose tissues of severely obese patients. Obesity. 2007;15:40–9.
Farooqi IS, O’Rahilly S. Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity. Nat Clin Pract Endocrinol Metab. 2008;4:569–77.
Burgos C, Carrodeguas JA, Moreno C, Altarriba J, Tarrafeta L, Barcelona JA, et al. Allelic incidente in several pig breeds of a missense variant of pig melanocortin-4 receptor (MC4R) gene associated with carcass and productive traits; its relation to IGF2 genotype. Meat Sci. 2006;73:144–50.
Lampert KP, Schmidt C, Fischer P, Volff JN, Hoffmann C, Muck J, et al. Determination of onset of sexual maturation and mating behavior by melanocortin receptor 4 polymorphisms. Curr Biol. 2010;20:1729–34.
Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev. 2010;31:506–43.
Pasquali R, Gambineri A. Metabolic effects of obesity on reproduction. Reprod Biomed Online. 2006;12:542–51.
Bellver J, Busso C, Pellicer A, Remohí J, Simón C. Obesity and assisted reproductive technology outcomes. Reprod Biomed Online. 2006;12:562–8.
Fedorcsák P, Dale PO, Storeng R, Ertzeid G, Bjercke S, Oldereid N, et al. Impact of overweight and underweight on assisted reproduction treatment. Hum Reprod. 2004;19:2523–8.
Metwally M, Ledger WL, Li TC. Reproductive endocrinology and clinical aspects of obesity in women. Ann NY Acad Sci. 2008;1127:140–6.
Rátky J, Brüssow KP, Egerszegi I, Torner H, Schneider F, Solti L, et al. Comparison of follicular and oocyte development and reproductive hormone secretion during the ovulatory period in Hungarian native breed, Mangalica, and Landrace gilts. J Reprod Develop. 2005;51:427–32.
Ovilo C, Fernandez A, Rodriguez MC, Nieto M, Silio L. Association of MC4R gene variants with growth, fatness, carcass composition and meat and fat quality traits in heavy pigs. Meat Sci. 2006;73:42–7.
Rátky J, Torner H, Egerszegi I, Schneider F, Sarlos P, Manabe N, et al. Ovarian activity and oocyte development during follicular development in pigs at different reproductive phases estimated by the repeated endoscopic method. J Reprod Dev. 2005;51:109–15.
Hazeleger W, Bouwman EG, Noordhuizen JP, Kemp B. Effect of superovulation induction on embryonic development on day 5 and subsequent development and survival after nonsurgical embryo transfer in pigs. Theriogenology. 2000;53:1063–70.
Gonzalez-Añover P, Encinas T, Gomez-Izquierdo E, Sanz E, Sanchez-Sanchez R, Gonzalez-Bulne A. Accuracy of in vivo and ex vivo ultrasonographic evaluation of ovarian follicles and corpora lutea in sows. Theriogenology. 2009;71:1433–9.
Hunter MG, Wiesak T. Evidence for and implications of follicular heterogeneity in pigs. J Reprod Fertil Suppl. 1990;40:163–77.
Yen HW, Ford JJ, Zimmerman DR, Johnson RK. Follicular development and maturation in gilts selected for an index of high ovulation rate and high prenatal survival. J Anim Sci. 2005;83:130–5.
Petters RM, Wells KD. Culture of pig embryos. J Reprod Fertil. 1993;48(Suppl):61–73.
Langendijk P, Dieleman SJ, van Dooremalen C, Foxcroft GR, Gerritsen R, Hazeleger W. LH and FSH secretion, follicle development and oestradiol in sows ovulating or failing to ovulate in an intermittent suckling regimen. Reprod Fert Develop. 2009;21:313–22.
Lopez-Bote C. Sustained utilization of the Iberian pig breed. Meat Sci. 1998;49:S17–27.
Gonzalez-Añover P, Encinas T, Torres-Rovira L, Pallares P, Muñoz-Frutos J, Gomez-Izquierdo E, Sanchez-Sanchez R, Gonzalez-Bulnes A. Ovulation rate, embryo mortality and intrauterine growth retardation in obese swine with gene polymorphisms for leptin and melanocortin receptors. Theriogenology (in press).
Gonzalez-Añover P, Encinas T, Sanz E, Letelier CA, Torres-Rovira L, de Mercado E, Pallares P, Sanchez-Sanchez R, Gonzalez-Bulnes A. Preovulatory follicle dynamics and ovulatory efficiency in sows with thrifty genotype and leptin resistance due to leptin receptor gene polymorphisms (Iberian pig). Gen Comp Endocrinol. 2010 (in press).
Fedorcsák P, Storeng R, Dale PO, Tanbo T, Torjesen P, Urbancsek J. Leptin and leptin binding activity in the preovulatory follicle of polycystic ovary syndrome patients. Scand J Clin Lab Invest. 2000;60:649–55.
Jungheim ES, Schoeller EL, Marquard KL, Louden ED, Schaffer JE, Moley KH. Diet-induced obesity model: abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology. 2010;151:4039–46.
Mermillod P, Oussaid B, Cognie Y. Aspects of follicular and oocyte maturation that affect the developmental potential of embryos. J Reprod Fertil Suppl. 1999;54:449–60.
Baird DT, McNeilly AS. Onadotrophic control of follicular development and function during the oestrous cycle in the ewe. J Reprod Fertil Suppl. 1981;30:129–33.
Baird DT. Factors regulating the growth of the preovulatory follicle in sheep and human. J Reprod Fertil. 1983;69:343–52.
Kelly CR, Kopf JD, Zimmerman DR. Characterization of antral follicle populations during the estrous cycle in pigs selected for ovulation rate. J Anim Sci. 1988;66:1230–5.
Foxcroft GR, Hunter MG. Basic physiology of follicular maturation in the pig. J Reprod Fertil Suppl. 1985;33:1–19.
Pallares P, Garcia-Fernandez RA, Criado LM, Letelier CA, Fernandez-Toro JM, Esteban D, et al. Substantiation of ovarian effects of leptin by challenging a mouse model of obesity/type 2 diabetes. Theriogenology. 2010;73:1088–95.
Gonzalez RR, Simon C, Caballero-Campo P, Norman R, Chardonnens D, Devoto L, et al. Leptin and reproduction. Hum Reprod Update. 2000;6:290–300.
Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, et al. Effects of gender, body composition, andmenopause on plasma concentrations of leptin. J Clin Endocrinol Metab. 1996;81:3424–7.
Shimizu H, Shimomura Y, Nakanishi Y, Futawatari T, Ohtani K, Sato N, et al. Estrogen increases in vivo leptin production in rats and human subjects. J Endocrinol. 1997;154:285–92.
Hong SC, Yoo SW, Cho GJ, Kim T, Hur JY, Park YK, et al. Correlation between estrogens and serum adipocytokines in premenopausal and postmenopausal women. Menopause. 2007;14:835–40.
Joo JK, Joo BS, Kim SC, Choi JR, Park SH, Lee KS. Role of leptin in improvement of oocyte quality by regulation of ovarian angiogenesis. Anim Reprod Sci. 2010;119:329–34.
Gregoraszczuk EL, Wojtowicz AK, Ptak A, Nowak K. In vitro effect of leptin on steroids’ secretion by FSH- and LH-treated porcine small, medium and large preovulatory follicles. Biol Reprod. 2003;3:227–39.
Ruiz-Cortes ZT, Martel-Kennes Y, Gevry NY, Downey BR, Palin MF, Murphy BD. Biphasic effects of leptin in porcine granulosa cells. Biol Reprod. 2003;68:789–96.
Przała J, Gregoraszczuk EL, Kotwica G, Stefańczyk-Krzymowska S, Ziecik AJ, Blitek A, et al. Mechanisms ensuring optimal conditions of implantation and embryo development in the pig. Reprod Biol. 2006;6:59–87.
Sandrock M, Schulz A, Merkwitz C, Schöneberg T, Spanel-Borowski K, Ricken A. Reduction in corpora lutea number in obese melanocortin-4-receptor-deficient mice. Reprod Biol Endocrinol. 2009;24(7):24.
Yu WH, Walczewska A, Karanth S, McCann SM. Nitric oxide mediates leptin-induced luteinizing hormone-releasing hormone (LHRH) and LHRH and leptin-induced LH release from the pituitary gland. Endocrinology. 1997;138:5055–8.
Bellver J, Melo MA, Bosch E, Serra V, Remohí J, Pellicer A. Obesity and poor reproductive outcome: the potential role of the endometrium. Fertil Steril. 2007;88:446–51.
Bellver J, Ayllón Y, Ferrando M, Melo M, Goyri E, Pellicer A, et al. Female obesity impairs in vitro fertilization outcome without affecting embryo quality. Fertil Steril. 2010;93:447–54.
Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–5.
Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–71.
Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–5.
Chua Jr SC, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, et al. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271:994–6.
Clément K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401.
Quinton ND, Lee AJ, Ross RJ, Eastell R, Blakemore AI. single nucleotide polymorphism (SNP) in the leptin receptor is associated with BMI, fat mass and leptin levels in postmenopausal Caucasian women. Hum Genet. 2001;108:233–6.
Yiannakouris N, Yannakoulia M, Melistas L, Chan JL, Klimis-Zacas D, Mantzoros CS. The Q223R polymorphism of the leptin receptor gene is significantly associated with obesity and predicts a small percentage of body weight and body composition variability. J Clin Endocrinol Metab. 2001;86:4434–9.
Paracchini V, Pedotti P, Taioli E. Genetics of leptin and obesity: a HuGE review. Am J Epidemiol. 2005;162:101–14.
Ranadive SA, Vaisse C. Lessons from extreme human obesity: monogenic disorders. Endocrinol Metab Clin North Am. 2008;37:733–51.
Ukkola O, Bouchard C. Role of candidate genes in the responses to long-term overfeeding: review of findings. Obes Rev. 2004;5:3–12.
Zabeau L, Defeau D, Van der Heyden J, Iserentant H, Vandekerckhove J, Tavernier J. Functional analysis of leptin receptor activation using a Janus kinase/signal transducer and activator of transcription complementation assay. Mol Endocrinol. 2004;18:150–61.
Acknowledgements
The authors gratefully thank ACOMPOR and CPP staff for skilled technical assistance. This work was supported by funds from INIA (project RZ07-010) and ITACyL (project PEP 2008/01043) under the collaborative project CC07-014.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
Differences in follicle dynamics and oocyte developmental competence between obese and lean genotypes sows, submitted to exogenous gonadotrophin stimulation.
Rights and permissions
About this article
Cite this article
Muñoz-Frutos, J., Encinas, T., Pallares, P. et al. Developmental competence of antral follicles and their oocytes after gonadotrophin treatment of sows with gene polymorphisms for leptin and melanocortin receptors (Iberian pig). J Assist Reprod Genet 28, 437–443 (2011). https://doi.org/10.1007/s10815-011-9535-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-011-9535-7