Abstract
Purpose
To optimize In vitro maturation (IVM) of quality oocytes for embryo production through IVF and SCNT.
Methods
Buffalo oocytes were in vitro matured in the presence of the pokeweed lectin (Phytolacca americana), a potent lymphocyte mitogen. Lectin was supplemented in TCM + 10% FBS at the doses of 0, 1, 5, 10, 15, 20 and 40 μg/ml and cumulus expansion and gene expression patterns were characterized.
Results
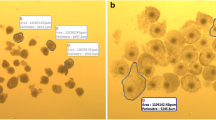
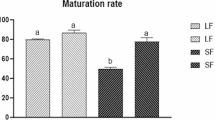
The degree of cumulus expansion in different lectin treatment levels improved from 1.1 at 1 Ag/ml level to 3.60 at 10 μg/ml level and then decreased in higher concentration 20 μg/ml (1.66) and 40 μg/ml (0.64). IVF embryos showed highest cleavage rate (88.8%) in 10 μg/ml lectin treatment. Expression of all mRNA transcript studied (Cx43, GDF 9, FGF-4 and Fibronectin) was positively correlated with cumulus expansion and polar body extrusion.
Conclusions
Mitogenic lectin supplemented maturation media improves oocyte quality for in vitro embryo production.




Similar content being viewed by others
References
Song JL, Wessel GM. How to make an egg: transcriptional regulation in oocytes. Differentiation. 2005;73:1–17. doi:10.1111/j.1432-0436.2005.07301005.x.
Adjaye J, Monk M. Transcription of homeoboxcontaining genes detected in cDNA libraries derived from human unfertilized oocytes and preimplantation embryos. Mol Hum Reprod. 2000;6:707–11. doi:10.1093/molehr/6.8.707.
Sirard MA, Richard F, Blondin P, Robert C. Contribution of the oocyte to embryo quality. Theriogenology. 2006;65:126–36. doi:10.1016/j.theriogenology.2005.09.020.
Sirard MA, Florman HM, Leibfried-Rutledge ML, Barnes FL, Sims ML, First NL. Timing of nuclear progression and protein synthesis necessary for meiotic maturation of bovine oocytes. Biol Reprod. 1989;40:1257–63. doi:10.1095/biolreprod40.6.1257.
Kastrop PM, Bevers MM, Destree OH, Kruip TA. Protein synthesis and phosphorylation patterns of bovine oocytes maturing in vivo. Mol Reprod Dev. 1991;29:271–5. doi:10.1002/mrd.1080290309.
Trimarchi JR, Keefe DL. Assessing the quality of oocytes derived from in vitro maturation: are we looking under the lamppost? Fertil Steril. 2006;85:839–40. doi:10.1016/j.fertnstert.2005.12.009.
Rizos D, Gutierrez-Adan A, Perez-Garnelo S, dela Fuente J, Boland MP, Lonergan P. Bovine embryo culture in the presence or absence of serum: implications for blastocyst development, cryotolerance, and messenger RNA expression. Biol Reprod. 2003;68:236–43. doi:10.1095/biolreprod.102.007799.
Eppig JJ. Gonadotropin stimulation of the expansion of cumulus oophori isolated from mice: general conditions for expansion in vitro. J Exp Zool. 1979;208:111–20. doi:10.1002/jez.1402080112.
Izadyar F, Zeinstra E, Bevers MM. Follicle stimulating hormone and growth hormone act differently on nuclear maturation while both enhance developmental competence of in vitro matured bovine oocytes. Mol Reprod Dev. 1998;51:339–45. doi:10.1002/(SICI)1098-2795(199811)51:3<339::AID-MRD14>3.0.CO;2-Y.
Zuelke KA, Brackett BG. Luteinizing hormone-enhanced in vitro maturation of bovine oocytes with and without protein supplementation. Biol Reprod. 1990;43:784–7. doi:10.1095/biolreprod43.5.784.
Calder MD, Caveney AN, Smith LC, Watson AJ. Responsiveness of bovine cumulus-oocyte-complexes (COC) to porcine and recombinant human FSH, and the effect of COC quality on gonadotropin receptor and Cx43 marker gene mRNAs during maturation in vitro. Reprod Biol Endocrinol. 2003;1:1–12. doi:10.1186/1477-7827-1-14.
Cyert MS, Kirschner MW. Regulation of MPF activity in vitro. Cell. 1988;53:185–95. doi:10.1016/0092-8674(88)90380-7.
Nicholsan GL. the interaction of lectins with animal cell surface. Int Rev Cytol. 1974;39:89–190. doi:10.1016/S0074-7696(08)60939-0.
Houghton FD. Role of gap junctions during early embryo development. Reproduction. 2005;129:129–35. doi:10.1530/rep. 1.00277.
Dragovic RA, Ritter LJ, Schulz SJ, Amato F, Armstrong DT, Gilchrist RB. Role of Oocyte-Secreted Growth Differentiation Factor 9 in the Regulation of Mouse Cumulus Expansion. Endocrinology. 2005;146:2798–806. doi:10.1210/en.2005-0098.
Lazzari G, Wrenzycki C, Herrmann D, Duchi R, Kruip T, Niemann H, et al. Cellular and molecular deviations in bovine in vitro-produced embryos are related to the large offspring syndrome. Biol Reprod. 2002;67:767–75. doi:10.1095/biolreprod.102.004481.
Goossens K, Van Soom A, Van Zeveren A, Favoreel H, Peelman LJ. Quantification of Fibronectin 1 (FN1) splice variants, including two novel ones, and analysis of integrins as candidate FN1 receptors in bovine preimplantation embryos. BMC Dev Biol. 2009;9:1. doi:10.1186/1471-213X-9-1.
Fagbohun CF, Downs SM. Maturation of the mouse oocyte-cumulus cell complex: stimulation by Lectins. Biol Reprod. 1990;42:413–23. doi:10.1095/biolreprod42.3.413.
Vanderhyden BC, Armstrong DT. Effects of gonadotropins and granulosa cell secretions on the maturation and fertilization of rat oocytes in vitro. Mol Reprod Dev. 1990;26:337–46. doi:10.1002/mrd.1080260408.
Madan ML, Singla SK, Chauhan MS, Manik RS. In vitro production and transfer of embryos in buffaloes. Theriogenology. 1994;41:139–43. doi:10.1016/S0093-691X(05)80059-7.
Brackett BG, Oliphant G. Capacitation of rabbit spermatozova in vitro. Biol Reprod. 1975;12:260–74. doi:10.1095/biolreprod12.2.260.
Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–93. doi:10.1677/jme.0.0250169.
Lonergan P, Rizos D, Gutierrez-Adan A, Moreira PM, de la Pintado B, FJ BMP. Temporal divergence in the pattern of messenger RNA expression in bovine embryos cultured from the zygote to blastocyst stage in vitro or in vivo. Biol Reprod. 2003;69:1424–31. doi:10.1095/biolreprod.103.018168.
Trounson A, Anderiesz C, Jones G. Maturation of human oocytes in vitro and their developmental competence. Reproduction. 2001;121:51–75. doi:10.1530/rep.0.1210051.
Modina S, Luciano AM, Vassena R, Baraldi-Scesi L, Lauria A, Gandolfi F. Oocyte developmental competence after in vitro maturation depends on the persistence of cumulus-oocyte communications which are linked to the intracellular concentration of cAMP. Ital J Anat Embryol. 2001;106:241–8.
DeSousa PA, Westhusin ME, Watson AJ. Analysis of variation in relative mRNA abundance for specific gene transcripts in single bovine oocytes and early embryos. Mol Reprod Dev. 1998;49:119–30. doi:10.1002/(SICI)1098-2795(199802) 49:2<119::AID-MRD3>3.0.CO;2-S.
Racedo S, Herrmann D, Wrenzycki C, Salamone D, Niemann H. Effects of follicle size and stage of maturation on mRNA expression in bovine in vitro matured oocytes. Reprod Fertil Dev. 2007;19:291. doi:10.1071/RDv19n1Ab352.
Farin CE, Rodriguez KF, Alexander JE, Hockney JE, Herrick JR, Kennedy-Stoskopf S. The role of transcription in EGF- and FSH-mediated oocyte maturation in vitro. Anim Reprod Sci. 2007;98:97–112. doi:10.1016/j.anireprosci.2006.10.007.
Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–70. doi:10.1016/0092-8674(91)90616-7.
George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–91.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pandey, A., Gupta, N. & Gupta, S. Improvement of in vitro oocyte maturation with lectin supplementation and expression analysis of Cx43, GDF-9, FGF-4 and Fibronectin mRNA transcripts in Buffalo (Bubalus bubalis). J Assist Reprod Genet 26, 365–371 (2009). https://doi.org/10.1007/s10815-009-9314-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-009-9314-x