Abstract
Purpose
MicroRNAs (miRNAs) are small non-coding RNA molecules that have been identified as potent regulators of gene expression. Recent studies indicate that miRNAs are involved in mammalian spermatogenesis but the mechanism of regulation is largely unknown.
Methods
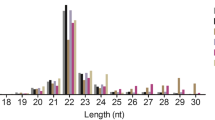
miRNA microarray was employed to compare miRNA expression profiles of testis tissues from immature rhesus monkey (Sample IR), mature rhesus monkey (Sample MR), and mature human (Sample MH). Real-time RT-PCR was uesd to confirm the changed miRNAs.
Results
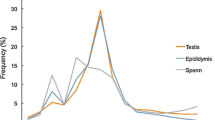
Twenty-six miRNAs were shared by samples IR/MR and IR/MH with differential expression patterns greater than three-fold difference. PicTar and TargetScan prediction tools predicted a number of target mRNAs, and some of these target genes predicted by miRNAs have been shown to associate with spermatogenesis.
Conclusions
Our results indicate that miRNAs are extensively involved in spermatogenesis and provide additional information for further studies of spermatogenetic mechanisms.



Similar content being viewed by others
References
Feng HL. Molecular biology of male infertility. Arch Androl. 2003;49:19–27. doi:10.1080/01485010290031556.
Hecht NB. Molecular mechanisms of male germ cell differentiation. Bioessays. 1998;20:555–61. doi:10.1002/(SICI) 1521-1878(199807) 20:7<555::AID-BIES6>3.0.CO;2-J.
Kleene KC. Patterns of translational regulation in the mammalian testis. Mol Reprod Dev. 1996;43:268–81. doi:10.1002/(SICI) 1098-2795(199602) 43:2<268::AID-MRD17>3.0.CO;2-#.
Ambros V. The functions of animal microRNAs. Nature. 2004;31:350–5. doi:10.1038/nature02871.
Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, et al. The Microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432:235–40. doi:10.1038/nature03120.
Yu Z, Raabe T, Hecht NB. MicroRNA Mirn122a reduces expression of the posttranscriptionally regulated germ cell transition protein 2 (Tnp2) messenger RNA (mRNA) by mRNA cleavage. Biol Reprod. 2005;73:427–33. doi:10.1095/biolreprod.105.040998.
Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNA from mouse. Curr Biol. 2002;12:735–9. doi:10.1016/S0960-9822(02) 00809-6.
Watanabe T, Takeda A, Tsukiyama T, Mise K, Okuno T, Sasaki H, et al. Identification and characterization of two novel classes of small RNAs in the mouse germline: retrotransposon-derived siRNAs in oocytes and germline small RNAs in testes. Genes Dev. 2006;20:1732–43. doi:10.1101/gad.1425706.
Ro S, Park C, Sanders KM, McCarrey JR, Yan W. Cloning and expression profiling of testis-expressed microRNAs. Dev Biol. 2007;311:592–602. doi:10.1016/j.ydbio.2007.09.009.
Braun RE. Post-transcriptional control of gene expression during spermatogenesis. Semin Cell Dev Biol. 1998;9:483–9. doi:10.1006/scdb.1998.0226.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi:10.1006/meth.2001.1262.
Peirson SN, Butler JN, Foster RG. Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res. 2003;31:e73. doi:10.1093/nar/gng073.
Lall S, Grün D, Krek A, Chen K, Wang YL, Dewey CN, et al. A genome-wide map of conserved microRNA targets in C. elegans. Curr Biol. 2006;16:460–71. doi:10.1016/j.cub.2006.01.050.
Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–98. doi:10.1016/S0092-8674(03) 01018-3.
Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, et al. Real-time quantification of microRNAs stem–loop RT–PCR. Nucleic Acids Res. 2005;33:e179. doi:10.1093/nar/gni178.
Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, et al. Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007;16:222–34.
Selbach M, Schwanhäusser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;7209:58–63. doi:10.1038/nature07228.
Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;7209:64–71. doi:10.1038/nature07242.
Scobey M, Bertera S, Somers J, Watkins S, Zeleznik A, Walker W. Delivery of a cyclic adenosine 3’, 5’-monophosphate response element-binding protein (CREB) mutant to seminiferous tubules results in impaired spermatogenesis. Endocrinology. 2001;142:948–54. doi:10.1210/en.142.2.948.
Granadino B, Arias-de-la-Fuente C, Pérez-Sánchez C, Párraga M, López-Fernández LA, del Mazo J, et al. Fhx (Foxj2) expression is activated during spermatogenesis and very early in embryonic development. Mech Dev. 2000;97:157–60. doi:10.1016/S0925-4773(00) 00410-X.
Chieffi P, Battista S, Barchi M, Di Agostino S, Pierantoni GM, Fedele M, et al. HMGA1 and HMGA2 protein expression in mouse spermatogenesis. Oncogene. 2002;21:3644–50. doi:10.1038/sj.onc.1205501.
Godmann M, Kromberg I, Mayer J, Behr R. The mouse Krüppel-like Factor 4 (Klf4) gene: four functional polyadenylation sites which are used in a cell-specific manner as revealed by testicular transcript analysis and multiple processed pseudogenes. Gene. 2005;361:149–56. doi:10.1016/j.gene.2005.07.025.
Saito H, Takeda K, Yasumoto K, Ohtani H, Watanabe K, Takahashi K, et al. Germ cell-specific expression of microphthalmia-associated transcription factor mRNA in mouse testis. J Biochem. 2003;134:143–50. doi:10.1093/jb/mvg122.
Hayashi T, Kageyama Y, Ishizaka K, Xia G, Kihara K, Oshima H. Requirement of Notch 1 and its ligand jagged 2 expressions for spermatogenesis in rat and human testes. J Androl. 2001;22:999–1011.
Connor F, Wright E, Denny P, Koopman P, Ashworth A. The Sry-related HMG box-containing gene Sox6 is expressed in the adult testis and developing nervous system of the mouse. Nucleic Acids Res. 1995;23:3365–72. doi:10.1093/nar/23.17.3365.
Wilkerson DC, Wolfe SA, Grimes SR. Sp1 and Sp3 activate the testis specific histone H1t promoter through the H1t/GC-box. J Cell Biochem. 2002;86:716–25. doi:10.1002/jcb.10265.
Cui Q, Yu Z, Purisima EO, Wang E. Principles of microRNA regulation of a human cellular signaling network. Mol Syst Biol. 2006;2:46–52. doi:10.1038/msb4100089.
Zhou Y, Ferguson J, Chang JT, Kluger Y. Inter- and intra-combinatorial regulation by transcription factors and microRNAs. BMC Genomics. 2007;8:396–405. doi:10.1186/1471-2164-8-396.
Yamamoto CM, Hikim AP, Lue Y, Portugal AM, Guo TB, Hsu SY, et al. Impairment of spermatogenesis in transgenic mice with selective overexpression of Bcl-2 in the somatic cells of the testis. J Androl. 2001;22:981–91.
Zheng S, Turner TT, Lysiak JJ. Caspase 2 activity contributes to the initial wave of germ cell apoptosis during the first round of spermatogenesis. Biol Reprod. 2006;74:1026–33. doi:10.1095/biolreprod.105.044610.
Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–4. doi:10.1126/science.1149460.
Nicchia GP, Frigeri A, Nico B, Ribatti D, Svelto M. Tissue distribution and membrane localization of aquaporin-9 water channel: evidence for sex-linked differences in liver. J Histochem Cytochem. 2001;49:1547–56.
Domeniconi RF, Orsi AM, Justulin LA Jr, Beu CC, Felisbino SL. Aquaporin 9 (AQP9) Localization in the Adult Dog Testis Excurrent Ducts by Immunohistochemistry. Anat Rec. 2007;290:1519–25. doi:10.1002/ar.20611.
Lopez P, Vidal F, Martin L, Lopez-Fernandez LA, Rual JF, Rosen BS, et al. Gene control in germinal differentiation: Rnf6, a transcription regulatory protein in the mouse sertoli cell. Mol Cell Biol. 2002;22:3488–96. doi:10.1128/MCB.22.10.3488-3496.2002.
Acknowledgments
We thank Dr Xuyang Liu (Laboratory of Ophthalmology, West China Hospital) for many helpful discussions during this work and Lei Chen (University of Oklahoma Health Sciences, Oklahoma City, OK) for his editorial assistance. This work was supported by National Program of High-tech Research and Development (863 Program) (grant no. 2008AA02Z102), National Nature Science Foundation of China (grant no. 90408025, 30500186 and 30770812) and Trans-Century Training Programme Foundation for the Talents by the Ministry of Education of China (grant no. NCET-07-0580). The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
This work, based on microRNA microarray assay and qRT-PCR assay, predicted genes associated with spermatogenesis by microRNA analysis.
Rights and permissions
About this article
Cite this article
Yan, N., Lu, Y., Sun, H. et al. Microarray profiling of microRNAs expressed in testis tissues of developing primates. J Assist Reprod Genet 26, 179–186 (2009). https://doi.org/10.1007/s10815-009-9305-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-009-9305-y