Abstract
Purpose
To analyze the gap junction proteins connexin 37 (Cx37) and connexin 43 (Cx43) after subcutaneous transplantation of cryopreserved mouse ovarian tissue.
Methods
Expression of gap junction genes was assessed by immunohistochemistry and real-time polymerase chain reaction (PCR) in transplanted cryopreserved ovarian tissue compared with that of normal ovarian tissue. Apoptosis of ovarian cells was evaluated by using the terminal deoxynucleotidyl transferase-mediated biotinylated deoxyuridine triphosphates nick end-labeling method.
Results
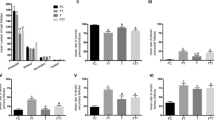
After subcutaneous transplantation, Cx37 and Cx43 mRNA and protein expression were significantly lower in cryopreserved than in normal ovarian tissue. Apoptosis was increased in granulosa cells from antral follicles of the cryopreserved tissue.
Conclusion
After cryopreservation and subcutaneous transplantation of ovarian tissue, proteins forming gap junctions between oocytes and granulosa cells are under-expressed compared with normal controls.




Similar content being viewed by others
References
Chambers SK, Chambers JT, Kier R, Peschel RE. Sequelae of lateral ovarian transposition in irradiated cervical cancer patients. Int J Radiat Oncol Biol Phys. 1991;20:1305–8.
Donnez J, Martinez-Madrid B, Jadoul P, Van Langendonckt A, Demylle D, Dolmans MM. Ovarian tissue cryopreservation and transplantation: a review. Hum Reprod Update. 2006;12:519–35. doi:10.1093/humupd/dml032.
Shamonki MI, Oktay K. Oocyte and ovarian tissue cryopreservation: indications, techniques, and applications. Semin Reprod Med. 2005;23:266–76. doi:10.1055/s-2005-872455.
Siebzehnrubl E. Cryopreservation of ovarian tissue to preserve female fertility - state of the art. Andrologia. 2003;35:180–1. doi:10.1046/j.1439-0272.2003.00552_10.x.
Ovarian tissue and oocyte cryopreservation. Fertil Steril. 2006;86:S142–7.
Poirot CJ, Martelli H, Genestie C, Golmard JL, Valteau-Couanet D, Helardot P, et al. Feasibility of ovarian tissue cryopreservation for prepubertal females with cancer. Pediatr Blood Cancer. 2007;49:74–8. doi:10.1002/pbc.21027.
Jadoul P, Donnez J, Dolmans MM, Squifflet J, Lengele B, Martinez-Madrid B. Laparoscopic ovariectomy for whole human ovary cryopreservation: technical aspects. Fertil Steril. 2007;87:971–5. doi:10.1016/j.fertnstert.2006.10.012.
Martinez-Madrid B, Camboni A, Dolmans MM, Nottola S, Van Langendonckt A, Donnez J. Apoptosis and ultrastructural assessment after cryopreservation of whole human ovaries with their vascular pedicle. Fertil Steril. 2007;87:1153–65. doi:10.1016/j.fertnstert.2006.11.019.
Martinez-Madrid B, Donnez J. Cryopreservation of intact human ovary with its vascular pedicle—or cryopreservation of hemiovaries? Hum Reprod. 2007;22:1795–6. author reply 1796–7. doi:10.1093/humrep/dem047.
Candy CJ, Wood MJ, Whittingham DG. Restoration of a normal reproductive lifespan after grafting of cryopreserved mouse ovaries. Hum Reprod. 2000;15:1300–4. doi:10.1093/humrep/15.6.1300.
Liu J, Van der Elst J, Van den Broecke R, Dhont M. Live offspring by in vitro fertilization of oocytes from cryopreserved primordial mouse follicles after sequential in vivo transplantation and in vitro maturation. Biol Reprod. 2001;64:171–8. doi:10.1095/biolreprod64.1.171.
Liu J, Van Der Elst J, Van Den Broecke R, Dumortier F, Dhont M. Maturation of mouse primordial follicles by combination of grafting and in vitro culture. Biol Reprod. 2000;62:1218–23. doi:10.1095/biolreprod62.5.1218.
Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–10. doi:10.1016/S0140-6736(04)17222-X.
Oktay K, Economos K, Kan M, Rucinski J, Veeck L, Rosenwaks Z. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. JAMA. 2001;286:1490–3. doi:10.1001/jama.286.12.1490.
Lee DM, Yeoman RR, Battaglia DE, Stouffer RL, Zelinski-Wooten MB, Fanton JW, et al. Live birth after ovarian tissue transplant. Nature. 2004;428:137–8. doi:10.1038/428137a.
Oktay K, Buyuk E, Veeck L, Zaninovic N, Xu K, Takeuchi T, et al. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;363:837–40. doi:10.1016/S0140-6736(04)15728-0.
Lee RK, Ho HY, Yu SL, Lu CH. Blastocyst development after cryopreservation and subcutaneous transplantation of mouse ovarian tissue. J Assist Reprod Genet. 2005;22:95–101. doi:10.1007/s10815-005-1499-z.
Yang HY, Cox SL, Jenkin G, Findlay J, Trounson A, Shaw J. Graft site and gonadotrophin stimulation influences the number and quality of oocytes from murine ovarian tissue grafts. Reproduction. 2006;131:851–9. doi:10.1530/rep.1.00916.
Choi J, Lee JY, Lee E, Yoon BK, Bae D, Choi D. Cryopreservation of the mouse ovary inhibits the onset of primordial follicle development. Cryobiology. 2007;54:55–62. doi:10.1016/j.cryobiol.2006.11.003.
Wolner-Hanssen P, Hagglund L, Ploman F, Ramirez A, Manthorpe R, Thuring A. Autotransplantation of cryopreserved ovarian tissue to the right forearm 4(1/2) years after autologous stem cell transplantation. Acta Obstet Gynecol Scand. 2005;84:695–8. doi:10.1111/j.0001-6349.2005.00654.x.
Gosden R, Newton H, Kim SS. The cryopreservation of human ovarian tissue. In: Kempers RD, Cohen J, Haney AF, Younger JB, editors. Fertility and reproductive medicine. Amsterdam: Elsevier; 1998. p. 615–20.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods Methods. 2001;25:402–8.
Yeh HI, Lai YJ, Lee YN, Chen YJ, Chen YC, Chen CC, et al. Differential expression of connexin43 gap junctions in cardiomyocytes isolated from canine thoracic veins. J Histochem Cytochem. 2003;51:259–66.
Yeh HI, Lu CS, Wu YJ, Chen CC, Hong RC, Ko YS, et al. Reduced expression of endothelial connexin37 and connexin40 in hyperlipidemic mice: recovery of connexin37 after 7-day simvastatin treatment. Arterioscler Thromb Vasc Biol. 2003;23:1391–7. doi:10.1161/01.ATV.0000083508.21989.15.
Kidder GM, Mhawi AA. Gap junctions and ovarian folliculogenesis. Reproduction. 2002;123:613–20. doi:10.1530/rep.0.1230613.
Carabatsos MJ, Sellitto C, Goodenough DA, Albertini DF. Oocyte-granulosa cell heterologous gap junctions are required for the coordination of nuclear and cytoplasmic meiotic competence. Dev Biol. 2000;226:167–79. doi:10.1006/dbio.2000.9863.
Goodenough DA, Simon AM, Paul DL. Gap junctional intercellular communication in the mouse ovarian follicle. Novartis Found Symp. 1999;219:226–35. discussion 235–40. doi:10.1002/9780470515587.ch14.
Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296:2178–80. doi:10.1126/science.1071965.
Simon AM, Goodenough DA, Li E, Paul DL. Female infertility in mice lacking connexin 37. Nature. 1997;385:525–9. doi:10.1038/385525a0.
Siebzehnrubl E, Kohl J, Dittrich R, Wildt L. Freezing of human ovarian tissue—not the oocytes but the granulosa is the problem. Mol Cell Endocrinol. 2000;169:109–11. doi:10.1016/S0303-7207(00)00362-2.
Kim SS, Yang HW, Kang HG, Lee HH, Lee HC, Ko DS, et al. Quantitative assessment of ischemic tissue damage in ovarian cortical tissue with or without antioxidant (ascorbic acid) treatment. Fertil Steril. 2004;82:679–85. doi:10.1016/j.fertnstert.2004.05.022.
Navarro-Costa P, Correia SC, Gouveia-Oliveira A, Negreiro F, Jorge S, Cidadao AJ, et al. Effects of mouse ovarian tissue cryopreservation on granulosa cell-oocyte interaction. Hum Reprod. 2005;20:1607–14. doi:10.1093/humrep/deh787.
Nottola SA, Camboni A, Van Langendonckt A, Demylle D, Macchiarelli G, Dolmans MM, et al. Cryopreservation and xenotransplantation of human ovarian tissue: an ultrastructural study. Fertil Steril. 2007; In press.
Fauque P, Ben Amor A, Joanne C, Agnani G, Bresson JL, Roux C. Use of trypan blue staining to assess the quality of ovarian cryopreservation. Fertil Steril. 2007;87:1200–7. doi:10.1016/j.fertnstert.2006.08.115.
Israely T, Nevo N, Harmelin A, Neeman M, Tsafriri A. Reducing ischaemic damage in rodent ovarian xenografts transplanted into granulation tissue. Hum Reprod. 2006;21:1368–79. doi:10.1093/humrep/del010.
Acknowledgements
This work was supported by grants from the National Science Council (NSC93-2314-B-195-011) and Mackay Memorial Hospital (MMH 9408), Taipei, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Robert Kuo-Kuang Lee and Sheng-Hsiang Li contributed equally to this work.
Capsule
Abnormally low expression of Cx37 on oocytes and Cx43 on granulosa cells in subcutaneously transplanted cryopreserved mouse ovaries may contribute to failure of oocyte maturation.
Rights and permissions
About this article
Cite this article
Lee, R.KK., Li, SH., Lu, CH. et al. Abnormally low expression of connexin 37 and connexin 43 in subcutaneously transplanted cryopreserved mouse ovarian tissue. J Assist Reprod Genet 25, 489–497 (2008). https://doi.org/10.1007/s10815-008-9264-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-008-9264-8