Abstract
Purpose To investigate follicle survival and developmental potential with IVF of cryopreserved, subcutaneously transplanted mouse ovarian tissue.
Methods Fresh and frozen mouse ovarian tissue was autologously transplanted into subcutaneous tissue. Two weeks after the transplantation, the morphology and histology of the fresh and frozen grafts were compared. Superovulation and IVF was performed to evaluate the fertility potential of the frozen ovarian graft.
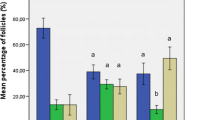
Results Both fresh and frozen grafts of ovarian tissue survived in 14 of 16 mice (88%). Morphologically, both types of grafts resembled fresh ovarian tissue and contained follicles at all stages of folliculogenesis. A total of 73% of follicles in fresh grafts and 62% in frozen grafts survived after transplantation compared with fresh ovarian tissue. Sixteen ICR mice underwent superovulation. A total of 56 oocytes from antral follicles were recovered from the subcutaneously transplanted cryopreserved ovarian tissue. Fourteen (25%) oocytes were in metaphase II stage, 6 were fertilized by IVF, and 2 progressed to the blastocyst stage.
Conclusions Cryopreservation and subcutaneous transplantation of ovarian tissue provides a possible means of fertility preservation. The main loss of follicles occurred during grafting rather than during freezing and thawing.
Similar content being viewed by others
References
N. Nugent, D. Deirow, PF, Brook, Y. Aubard, RG. Gosden, Transplantation in reproductive medicine: Previous experience, present knowledge and future prospects. Hum Reprod Update 1997;3:267– 280
Candy CJ, Wood MJ, Whittingham DG: Restoration of a normal reproductive lifespan after grafting of cryopreserved mouse ovaries. Hum Reprod 2000;15:1300– 1304
Liu J, Van der Elst J, Van den Broecke R, Dumortier F, Dhont M: Maturation of mouse primordial follicles by combination of grafting and in vitro culture. Biol Reprod 2000;62:1218– 1223
Liu J, Van der Elst J, Van den Broecke R, Dhont M: Live offspring by in vitro fertilization of oocytes from cryopreserved primordial mouse follicles after sequential in vivo transplantation and in vitro maturation. Biol Reprod 2001;64:171– 178
Hongbo W, Mooney S, Wen Y, Behr B, Polan ML: Follicle development in grafted mouse ovaries after cryopreservation and subcutaneous transplantation. Am J Obstet Gynecol 2002;187:370– 374
Oktay K, Economos K, Kan M, Rucinski J, Veeck L, Rosenwaks Z: Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. JAMA. 2001;286:1490– 1493
Gosden R, Newton H, Kim SS: The cryopreservation of human ovarian tissue. In Fertility and Reproductive Medicine. RD Kempers, J Cohen, AF Haney, JB Younger (eds), Amsterdam, Elsevier, 1998, pp 615– 620
Porcu, E: Oocyte cryopreservation. In Textbook of Assisted Reproductive Techniques: Laboratory and Clinical Perspectives, DK Gardner, A Weissman, CM Howles, Z Shoham (eds), London, UK, Martin Dunitz, 2001
Gosden RG, Byatt-Smith JG: Oxygen concentration gradient across the ovarian follicle epithelium: Model, predictions and implications. Hum Reprod 1986;1:65– 68
Nisolle M, Casanas-Roux F, Qu J, Motta P, Donnez J: Histologic and ultrastructural evaluation of fresh and frozen-thawed human ovarian xenografts in nude mice. Fertil Steril 2000;74:122– 129
Baird DT, Webb R, Campbell BK, Harkness LM, Gosden RG: Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at −196∘C. Endocrinology 1999;140:462– 471
Salle B, Demirci B, Franck M, Berthollet C, Lomage J: Long-term follow-up of cryopreserved hemi-ovary autografts in ewes: Pregnancies, births, and histologic assessment. Fertil Steril 2003;80(1):172– 177
Oktay K, Aydin BA, Karlikaya G: A technique for laparoscopic transplantation of frozen-banked ovarian tissue. Fertil Steril 2001;75:1212– 1216
Oktay K, Newton H, Mullan J, Gosden RG: Development of human primordial follicles to antral stages in SCID/hpg mice stimulated with follicle stimulating hormone. Hum Reprod 1998;13:1133– 1138
Oktay K, Newton H, Gosden RG: Transplantation of cryopreserved human ovarian tissue results in follicle growth initiation in SCID mice. Fertil Steril 2000;73:599– 603
Aubard Y, Piver P, Cognie Y, Fermeaux V, Poulin N, Driancourt MA: Orthotopic and heterotopic autografts of frozen-thawed ovarian cortex in sheep. Hum Reprod 1999;14:2149– 2154
Jeremias E, Bedaiwy MA, Nelson D, Biscotti CV, Falcone T: Assessment of tissue injury in cryopreserved ovarian tissue. Fertil Steril 2003;79(3):651– 653
Laschke MW, Menger MD, Vollmar B: Cryopreservation does not affect neovascularization of freely transplanted ovarian follicles. Fertil Steril 2003;79(6):1458– 1460
Fabbri R, Venturoli S, D’Errico A, Iannascoli C, Gabusi E, Valeri B, Seracchioli R, Grigioni WF: Ovarian tissue banking and fertility preservation in cancer patients: Histological and immunohistochemical evaluation. Gynecol Oncol 2003;89:259– 266
Hogan B, Beddington R, Constantini F, Lacy E: Inducing superovulation. In Manipulating the Mouse Embryo. A Laboratory Manual. B Hogan, R Beddington, F Constantini, E Lacy (eds), Taipei, Taiwan, Republic of China, Cold Spring Harbor Laboratory Press, 1994, pp 130– 132
Oktay K, Buyuk E, Veeck L, Zaninovic N, Xu K, Takeuchi T, Opsahl M, Rosenwaks Z: Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet 2004;363:837– 840
Nugent D, Newton H, Gallivan L, Gosden RG: Protective effect of vitamin E on ischaemia-reperfusion injury in ovarian grafts. J Reprod Fertil 1998;114:341– 346
Schnorr J, Oehninger S, Toner J, Hsiu J, Lanzendorf S, Williams R, Hodgen G: Functional studies of subcutaneous ovarian transplants in non-human primates: Steroidogenesis, endometrial development, ovulation, menstrual patterns and gamete morphology. Hum Reprod 2002;17:612– 619
Liu J, Van der Elst J, Van den Broecke R, Dhont M: Early massive follicle loss and apoptosis in heterotopically grafted newborn mouse ovaries. Hum Reprod 2002;17:605– 611
Gosden RG. Ovulation 1: Oocyte development throughout life. In: Gametes— the oocytes. JG Grudzinskas, JL Yovich (eds), Cambridge, UK, Cambridge University Press, 1995, pp 119– 149
Matzuk MM, Burns KH, Viveiros MM, Eppig JJ: Intercellular communication in the mammalian ovary: Oocytes carry the conversation. Science 2002;296(5576):2178– 2180
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, R.KK., Ho, HY., Yu, SL. et al. Blastocyst Development After Cryopreservation and Subcutaneous Transplantation of Mouse Ovarian Tissue. J Assist Reprod Genet 22, 95–101 (2005). https://doi.org/10.1007/s10815-005-1499-z
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10815-005-1499-z