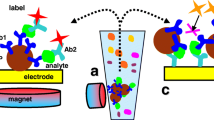
Fe3O4 nanoparticles were first modified with tetraethoxylsilane to form Fe3O4/SiO2 nanoparticles, followed by the addition of 3-aminopropyltriethoxysilane and 3-thiolpropyltriethoxysilane to introduce –NH2 and –SH groups to the surface of Fe3O4/SiO2 nanoparticles. Gold nanoparticles were further assembled on the surface of Fe3O4/SiO2 via the electrostatic adsorption of –NH2 and the Au–S bond to produce stable core–shell Fe3O4/SiO2/Au gold/magnetic nanoparticles. These Fe3O4/SiO2/Au gold/magnetic nanoparticles were characterized by a variety of techniques such as transmission electron microscopy (TEM) and energy dispersive X-ray spectroscopy (EDX), and afterwards conjugated with tetrodotoxin antibodies (Ab) and used as a Raman active substrate (Fe3O4/SiO2/Au–Ab) with Rhodamine B (RhB)-labeled tetrodotoxin antibody as a Raman reporter (Ab–RhB). Upon mixing these reagents with tetrodotoxin (TTX), a sandwich complex [Fe3O4/SiO2/Au–Ab···TTX···Ab–RhB] was generated due to the specific antibody–antigen interactions. The immunocomplex was subsequently separated by an externally applied magnetic source and concentrated into a pellet point, where the laser interrogation of the pellet produced a strong signal characteristic of RhB. The logarithmic intensity of the signal was found to be proportional to the concentration of TTX with a limit of detection of 0.01 μg/mL and a detection linearity range of ~ 0.01–0.5 μg/mL. The established method eliminates the complicated procedures of traditional centrifuging, column separation, and incubation and achieves a rapid detection of tetrodotoxin with improved detection sensitivity.
Similar content being viewed by others
References
E. P. Ragelis, J. Assoc. Chem., 65, 327–331 (1982).
A. S. May, Chem. Ind., 24, 982–984 (1982).
T. Yasumoto, M. Nakamura, Y. Oshima, and J. Takahata, Bull. Jpn. Soc. Sci. Fish., 48, 1481–1483 (1982).
J. Huang and M. J. Yan, Chem. Biotechnol. Lett., 17, 998–1000 (2006).
T. Nakayama and S. Tetrakawa, Anal. Biochem., 126, 153–155 (1982).
T. Yasumato and T. Michishita, Agric. Biol. Chem., 49, 3077–3088 (1985).
M. Fleischmann, P. J. Hendra, and A. J. Mcquillan, Chem. Phys. Lett., 26, 163–166 (1974).
D. L. Jeanmaire and R. P. V. Duyne, J. Electroanal. Chem., 84, 1–20 (1977).
M. G. Albrecht and J. A. Creighton, J. Am. Chem., 99, 5215–5217 (2002).
N. P. Pieczonka and R. F. Acora, Chem. Soc. Rev., 37, 946–954 (2008).
X. Y. Zhang, J. Zhao, A. V. Whitney, J. W. Elan, and R. P. Duyne, J. Am. Chem. Soc., 128, 10304–10309 (2006).
M. Culha, D. Stokes, L. R. Allain, and T. Vo-Dinh, Anal. Chem., 75, 6196–6201 (2003).
Y. C. Cao, R. Jia, and C. A. Mirkin, Science, 297, 1536–1540 (2002).
T. Park, S. Lee, G. H. Seong, J. Choo, E. K. Lee, Y. S. Kim, W. H. Ji, S. Y. Hwang, and D. G. Gweon, Lab Chip, 5, 437–442 (2005).
M. Y. Sha, S. G. Penn, R. G. Freeman, and W. E. Doering, J. Nanobiotech., 3, 23–30 (2007).
Y. C. Cao, R. Jia, J. M. Nam, C. S. Thaxton, and C. A. Mirkin, J. Am. Chem. Soc., 125, 14676–14677 (2003).
J. D. Driskell, J. M. Uhlenkamp, R. J. Lipert, and M. D. Porter, Anal. Chem., 79, 4141–4148 (2007).
J. L. Gong, Y. Liang, Y. Huang, J. W. Chen, J. H. Jiang, G. L. Shen, and R. Q. Yu, Biosens. Bioelectron., 22, 1501–1507 (2007).
J. Neng, M. H. Harpster, H. Zhang, J. O. Mecham, W. C. Wilson, and P. Johnson, Biosens. Bioelectron., 26, 1009 (2010).
J. Neng, M. H. Harpster, W. C. Wilson, and P. Johnson, Biosens. Bioelectron., 41, 316 (2013).
A. P. Craig, A. S. Franca, and J. Irudayaraj, Annu. Rev. Food. Sci. Technol., 4, 369–380 (2013).
J. Zheng and L. He, Comp. Rev. Food. Sci. Food Safety, 13, 317–329 (2014).
Y. L. Wang, S. Ravindranath, and J. Irudayaraj, Anal. Bioanal. Chem., 399, 1271–1278 (2011).
G. H. Mirzabe and A. R. Keshtkar, J. Ind. Eng. Chem., 26, 277–285 (2015).
G. Frens, Nature, 241, 20–22 (1972).
P. Boolchand, M. Jin, D. I. Novita, and S. Chakravarty, J. Raman Spectrosc., 38, 660–672 (2007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Abstract of article is published in Zhurnal Prikladnoi Spektroskopii, Vol. 85, No. 1, p. 169, January–February, 2018.
Rights and permissions
About this article
Cite this article
Neng, J., Wang, X., Jia, K. et al. Rapid Detection of Tetrodotoxin Using Surface-Enhanced Raman Spectroscopy and Fe3O4/SiO2/Au Gold/Magnetic Nanoparticles. J Appl Spectrosc 85, 160–165 (2018). https://doi.org/10.1007/s10812-018-0627-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-018-0627-3