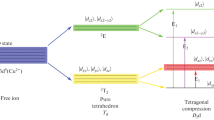
The spin Hamiltonian parameters (SHPs), i.e., g factors and hyperfine structure constants, and local structures are theoretically studied by analyzing tetragonally elongated 3d9 clusters for Cu2+ in xK2SO4–(50 – x)Na2SO4–50ZnSO4 glasses with various K2SO4 concentrations x. The concentration dependences of the SHPs are attributed to the parabolic decreases of the cubic field parameter Dq, orbital reduction factor k, relative tetragonal elongation ratio τ, and core polarization constant κ with x. The [CuO6]10– clusters are found to undergo significant elongations of about 17% due to the Jahn–Teller effect. The calculated cubic field splittings and the SHPs at various concentrations agree well with the experimental data.
Similar content being viewed by others
References
S. Chaguetmia, A. Boutarfaia, and M. Poulain, Energy Proc., 74, 470–476 (2015).
K. J. Rao, Bull. Mater. Sci., 2, 357–380 (1980).
R. Rama Kumar, A. S. Rao, and B. C. Venkata Reddy, Opt. Mater., 4, 723–728 (1995).
R. Rama Kumar, A. Srinivasa Rao, and B. C. Venkata Reddy, Transition Metal Chem., 21, 390–392 (1996).
G. L. Narendra, B. Sreedhar, J. Lakshmana Rao, and S. V. J. Lakshman, J. Mater. Sci., 26, 5342–5346 (1991).
R. Rama Kumar and B. C. Venkata Reddy, Radiat. Eff. Defects Solids, 147, 293–302 (1999)
L. H. Xie and Y. Y. Yeung, Appl. Magn. Reson., 40, 441–448 (2011).
A. Srinivasa Rao, J. Lakshmana Rao, R. Ramakrishna Reddy, and T. V. Ramakrishna Rao, Opt. Mater., 4, 717–721 (1995).
R. R. Kumar, A. K. Bhatnagar, and B. C. V. Reddy, Solid State Commun., 114, 493–497 (2000).
T. H. Chen and Y. Wu, P. Luo, Radiat. Eff. Defects Solids, 162, 633–636 (2007).
A. Abragam and B. Bleaney, Electron Paramagnetic Resonance of Transition Ions, Oxford University Press, London (1986).
A. S. Chakravarty, Introduction to the Magnetic Properties of Solids, Wiley-Interscience, New York (1980).
Y. V. Yablokov and T. A. Ivanova, Coord. Chem. Rev., 190–192, 1255–1267 (1999).
S.O. Graham and R.L. White, Phys. Rev. B, 10, 4505–4509 (1974).
S. Y. Wu, H. M. Zhang, P. Xu, and S. X. Zhang, Spectrochim. Acta A, 75, 230–234 (2010).
H. M. Zhang, S. Y. Wu, M. Q. Kuang, Z. H. Zhang, J. Phys. Chem. Solids, 73, 846–850 (2012).
D. J. Newman and B. Ng, Rep. Prog. Phys., 52, 699–763 (1989).
H. M. Zhang, S. Y. Wu, G. D. Lu, Z. H. Zhang, and L. H. Wei, Radiat. Eff. Defects Solids, 163, 789–794 (2008).
S. Y. Wu, H. N. Dong, H. M. Zhang, and G. D. Lu, Phys. Scr., 77, 025703(1–7) (2008).
Y. X. Hu, S. Y. Wu, and X. F. Wang, Philos. Mag. A, 90, 1391–1400 (2010).
S. Y. Wu and H. N. Dong, J. Alloys Compd., 451, 248–250 (2008).
X. F. Wang, S. Y. Wu, Z. H. Zhang, L. H. Wei, and Y. X. Hu, Mod. Phys. Lett. B, 22, 1381–1387 (2008).
M. A. Hassan, M. Farouk, A. H. Abdulah, I. Kashef, and M. M. Elokr, J. Alloys Compd., 539, 233–236 (2012).
C. K. Jørgensen, Absorption Spectra and Chemical Bonding in Molecules, 2nd ed., Pergamon Press Oxford, London, New York, and Paris (1964).
J. S. Griffith, The Theory of Transition-Metal Ions, Cambridge University Press, London (1964).
B. R. McGarvey, J. Phys. Chem., 71, 51–67 (1967).
A. Abragam and M. H. I. Pryce, Proc. Roy. Soc. (London) A, 206, 164–172 (1951).
A. W. Addison, in: Copper Coordination Chemistry: Biochemical and Inorganic Perspectives, Eds. K. D. Karlin and J. Zubieta, Adenine Press, New York (1983).
Y. Wu and Z. Chen, Radiat. Eff. Defects Solids, 169, 485–489 (2014).
M. Q. Kuang, S. Y. Wu, G. L. Li, and X. F. Hu, Mol. Phys., 113, 698–702 (2015).
H. M. Zhang and X. Wan, J. Non-Cryst. Solids, 361, 43–46 (2013).
J. L. Rao, G. Sivaramaiah, and O. Gopal, Physica B, 349, 206–213 (2004).
R. V. S. S. N. Ravikumar, R. Komatsu, K. Ikeda, A. V. Chandrasekhar, L. Ramamoorthy, B. J. Reddy, Y. P. Reddy, and P. S. Rao, Physica B, 334, 398–402 (2003).
G. Ramadevudu, M. Shareefuddin, N. S. Bai, M. L. Rao, and M. N. Chary, J. Non-Cryst. Solids, 278, 205–212 (2000).
Y. Q. Xu, S. Y. Wu, M. Q. Kuang, X. F. Hu, C. C. Ding, J. Non-Cryst. Solids, 432, 535–539 (2016).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Abstract of article is published in Zhurnal Prikladnoi Spektroskopii, Vol. 85, No. 1, p. 168, January–February, 2018.
Rights and permissions
About this article
Cite this article
Ding, CC., Wu, SY., Xu, YQ. et al. Evaluation of Spin Hamiltonian Parameters and Local Structure of Cu2+-doped Ion in xK2SO4–(50 – x)Na2SO4–50ZnSO4 Glasses with Various K2SO4 Concentrations. J Appl Spectrosc 85, 155–159 (2018). https://doi.org/10.1007/s10812-018-0626-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-018-0626-4