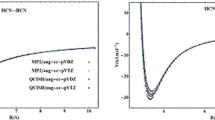
Previously calculated multidimensional potential-energy surfaces of the MeOH monomer and dimer, water dimer, malonaldehyde, formic acid dimer, free pyridine-N-oxide/trichloroacetic acid complex, and protonated water dimer were analyzed. The corresponding harmonic potential-energy surfaces near the global minima were constructed for series of clusters and complexes with hydrogen bonds of different strengths based on the behavior of the calculated multidimensional potential-energy surfaces. This enabled the introduction of an obvious anharmonicity parameter for the calculated potential-energy surfaces. The anharmonicity parameter was analyzed as functions of the size of the analyzed area near the energy minimum, the number of points over which energies were compared, and the dimensionality of the solved vibrational problem. Anharmonicity parameters for potential-energy surfaces in complexes with strong, medium, and weak H-bonds were calculated under identical conditions. The obtained anharmonicity parameters were compared with the corresponding diagonal anharmonicity constants for stretching vibrations of the bridging protons and the lengths of the hydrogen bridges.
Similar content being viewed by others
References
J. Ischtwan and M. Collins, J. Chem. Phys., 100, 8080–8088 (1994).
T. Ho and H. Rabitz, J. Chem. Phys., 104, 2584–2597 (1996).
P. R. P. Barreto, A. F. Albernaz, and F. Palazzetti, Int. J. Quantum Chem., 112, 834–847 (2012).
E. J. Saavedra, S. A. A. Fernando, D. Suvire, M. A. Zamora, M. L. Freile, and R. D. Enriz, Int. J. Quantum Chem., 112, 2382–2391 (2012).
Yu. N. Panchenko, V. I. Pupyshev, and A. V. Abramenkov, J. Mol. Struct., 130, 355–359 (1985).
Yu. N. Panchenko, V. I. Pupyshev, and A. V. Abramenkov, J. Mol. Struct., 140, 87–92 (1985).
E. Van Leuken, G. Brocks, and P. E. S. Wormer, Chem. Phys., 110, 365–373 (1986).
N. B. Balabanov and K. A. Peterson, J. Chem. Phys., 120, 6585–6592 (2004).
R. Prosmiti, S. Lopez-Lopez, and A. Garcia-Vela, J. Chem. Phys., 120, 6471–6477 (2004).
Y. Zhou and D. Xiea, J. Chem. Phys., 123, 134323 (2005).
B. Lowe, M. Pilant, and W. Rundell, SIAM J. Math. Anal., 23, 482–504 (1992).
R. Fabiano, R. Knobel, and B. Lowe, IMA J. Numer. Anal., 15, 75–88 (1995).
H. V. Von Geramb, Quantum Inversion Theory and Applications, Springer, New York (1994).
T. Ho and H. J. Rabitz, Phys. Chem., 97, 13447–13456 (1993).
T. Ho, H. Rabitz, S. Choi, and M. J. Lester, Chem. Phys., 104, 1187–1202 (1996).
D. Zhang and J. Light, J. Chem. Phys., 103, 9713–9720 (1995).
R. Baer and R. Kosloff, J. Phys. Chem., 99, 2534–2545 (1995).
M. Shapiro, J. Phys. Chem., 100, 7859–7866 (1996).
Z. Lu and H. Rabitz, Phys. Rev. A: At., Mol., Opt. Phys., 52, 1961–1967 (1995).
W. Zhu and H. Rabitz, J. Chem. Phys., 111, 472–480 (1999).
K. P. Lawley, I. Prigogine, and S. A. Rice, Ab initio Methods in Quantum Chemistry, Advances in Chemical Physics, 67, 69, Wiley, New York (1987).
L. Lain, A. Torre, M. Reguero, and C. Valdemoro, J. Mol. Struct.: THEOCHEM, 287, 47–53 (1993).
C. Valdemoro, M. Reguero, and L. Lain, Chem. Phys. Lett., 147, 219–222 (1988).
M. Reguero and C. Valdemoro, Basic Aspects of Quantum Chemistry, Elsevier, Amsterdam (1989).
I. Absar and A. J. Coleman, Chem. Phys. Lett., 39, 609–611 (1976).
D. J. Wales, Science, 293, No. 5537, 2067–2070 (2001).
D. J. Wales, J. Chem. Phys., 142, 130901 (2015).
D. Asenjo, J. D. Stevenson, D. J. Wales, and D. Frenkel, J. Phys. Chem. B, 117, 12717–12723 (2013).
M. T. Oakley, R. L. Johnston, and D. Wales, Phys. Chem. Chem. Phys., 15, 3965–3976 (2013).
W. Hermoso, N. B. Jaufeerally, P. Ramasami, and F. R. Ornellas, Int. J. Quantum Chem., 113, 112–118 (2013).
A. B. McCoy, Int. J. Quantum Chem., 113, 366–374 (2013).
F. Rao and A. Caflisch, J. Mol. Biol., 342, 299–306 (2004).
F. Noe and S. Fischer, Curr. Opin. Struct. Biol., 18, 154–162 (2008).
D. A. Evans and D. J. Wales, J. Chem. Phys., 118, 3891–3897 (2003).
G. S. Maciel, P. R. P. Barreto, F. Palazzetti, A. Lombardi, and V. Aquilanti, J. Chem. Phys., 129, 164302 (2008).
G. A. Pitsevich, A. E. Malevich, V. Sablinskas, I. Yu. Doroshenko, V. E. Pogorelov, E. N. Kozlovskaya, and V. Balevicius, Vest. Found. Fund. Res. [Vestn. Fonda Fundam. Issled.], 63, No. 1, 80–87 (2013).
G. A. Pitsevich, A. Malevich, I. Yu. Doroshenko, E. N. Kozlovskaya, V. Ye. Pogorelov, V. Shablinskas, and V. Balevichus, Spectrochim. Acta, Part A, 120, 585–594 (2014).
G. A. Pitsevich, A. Malevich, E. N. Kozlovskaya, I. Yu. Doroshenko, V. E. Pogorelov, V. Shablinskas, and V. Balevichus, Spectrochim. Acta, Part A, 145, 384–393 (2015).
G. A. Pitsevich, A. E. Malevich, E. N. Kozlovskaya, and U. U. Sapeshko, J. Spectrosc. Dyn., 4, 25 (2014).
G. Rauhut, J. Chem. Phys., 121, 9313–9322 (2004).
S. K. Burger and P. W. Ayers, J. Chem. Phys., 132, 234110 (2010).
M. R. Peterson, I. G. Csizmadia, and R. W. Sharpe, J. Mol. Struct.: THEOCHEM, 94, 127–135 (1983).
P. Gomez, M. Fernandez, L. Sese, and V. Botella, J. Mol. Struct., 142, 315–318 (1986).
G. L. Sosa, N. Peruchena, R. H. Contreras, and E. A. Castro, J. Mol. Struct.: THEOCHEM, 401, 77–85 (1997).
X. Luo and P. G. Mezey, Int. J. Quantum Chem., 41, 557–579 (1992).
N. J. Wright and R. B. Gerber, J. Chem. Phys., 112, 2598–2604 (2000).
B. Temelso and G. C. Shields, J. Chem. Theory Comput., 7, 2804–2817 (2011).
A. G. Csaszar, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2, 273–289 (2012).
V. Barone, M. Biczysko, and J. Bloino, Phys. Chem. Chem. Phys., 16, 1759–1787 (2014).
G. M. Chaban, J. O. Jung, and R. B. Gerber, J. Phys. Chem. A, 104, 2772–2779 (2000).
P. Meier, D. Oschetzki, R. Berger, and G. Rauhut, J. Chem. Phys., 140, 184111 (2014).
B. Njegic and M. S. Gordon, J. Chem. Phys., 129, 164107 (2008).
Z. Latajka, K. Morokuma, H. Ratajczak, and W. J. Orville-Thomas, J. Mol. Struct.: THEOCHEM, 135, 429–434 (1986).
Z. Latajka, K. Morokuma, H. Ratajczak, and W. J. Orville-Thomas, J. Mol. Struct.: THEOCHEM, 146, 263–266 (1986).
Y. Bouteiller, Z. Latajka, H. Ratajczak, and S. Scheiner, J. Chem. Phys., 94, 2956–2961 (1991).
Y. Bouteiller and Z. Latajka, J. Mol. Struct., 322, 175–180 (1994).
Y. Bouteiller and Z. Latajka, J. Chem. Phys., 97, 145–149 (1992).
B. Silvi, R. Wieczorek, Z. Latajka, M. E. Alikhani, A. Dkhissi, and Y. Bouteiller, J. Chem. Phys., 111, 6671–6678 (1999).
Y. G. Smeyers, J. Mol. Struct., 107, 3–21 (1984).
S. A. Manson, M. M. Law, I. A. Atkinson, and G. A. Thomson, Phys. Chem. Chem. Phys., 8, 2855–2865 (2006).
T. Xie and J. M. Bowman, J. Chem. Phys., 117, 10487–10493 (2002).
E. Matito, D. Toffoli, and O. Christiansen, J. Chem. Phys., 130, 134104 (2009).
S. Rampino, J. Phys. Chem. A, 120, 4683–4692 (2016).
M. S. Schuurman, W. D. Allen, P. V. R. Schleyer, and H. F. Schaefer III, J. Chem. Phys., 122, 104302 (2005).
F. Calvo, J. P. K. Doye, and D. J. Wales, J. Chem. Phys., 115, 9627–9636 (2001).
P. Botschwina, J. Chem. Soc., Faraday Trans. 2, 84, No. 9, 1263–1276 (1988).
G. A. Pitsevich, A. Malevich, E. N. Kozlovskaya, Yu. I. Doroshenko, V. Shablinskas, V. E. Pogorelov, D. Dovgal’, and V. Balevicius, Vib. Spectrosc., 79, 67–75 (2015).
G. Pitsevich, A. Malevich, E. Kozlovskaya, E. Mahnach, I. Doroshenko, V. Pogorelov, L. G. M. Pettersson, V. Sablinskas, and V. Balevicius, J. Phys. Chem. A, 121, 2151–2165 (2017).
S. Califano, Vibrational States, Wiley, New York (1976).
I. M. Mills, in: K. N. Rao and C. W. Mathews (Eds.), Molecular Spectroscopy: Modern Research, Academic Press, New York (1972).
Mathematica, Wolfram Research Inc.; http://www.wolfram.com/mathematica/
G. Pitsevich, A. Malevich, E. Kozlovskaya, E. Shalamberidze, I. Doroshenko, V. Pogorelov, E. Mahnach, V. Sapeshko, and V. Balevicius, J. Mol. Struct., 1139, 328–332 (2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Prikladnoi Spektroskopii, Vol. 84, No. 6, pp. 845–855, November–December, 2017.
Rights and permissions
About this article
Cite this article
Kozlovskaya, E.N., Doroshenko, I.Y., Pogorelov, V.E. et al. Comparison of Degrees of Potential-Energy-Surface Anharmonicity for Complexes and Clusters with Hydrogen Bonds. J Appl Spectrosc 84, 929–938 (2018). https://doi.org/10.1007/s10812-018-0567-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-018-0567-y