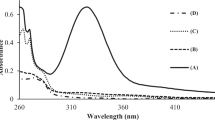
2-(5-Bromo-2-pyridylazo)-5-diethylaminophenol (5-Br-PADAP) is a highly sensitive chromogenic reagent that can react with most of the transition and alkaline earth metals. The Ni(II)-5-Br-PADAP complex is more stable than other metal–5-Br-PADAP complexes. In the presence of seignette salt, ethylenediaminetetraacetic acid (EDTA) can decompose most of the 5-Br-PADAP complexes with metals except for iron, cobalt, and nickel. Cetrimonium bromide (CTMAB) as a sensitizer for the color reaction forms a ternary complex with nickel and 5-Br-PADAP with maximum absorption wavelength at 561 nm. CTMAB can significantly improve the sensitivity and selectivity of nickel determination, as well as the stability and solubility of compounds. In this study, the determination of trace nickel in natural water samples was performed by flow injection analysis. The calibration lines were established in the range of 0–200 μg/l of nickel (n ≥ 3), and the limit of detection was 0.093 μg/l. The relative standard deviation was 2.55% for the determination of 25 μg/l nickel (n ≥ 20). The recoveries of this method ranged from 91.0 to 101% for environmental water samples. A large amount of aluminum, calcium, cadmium, copper, bicarbonate, magnesium, zinc, and iron, except for cobalt, did not interfere with the determination of nickel.
Similar content being viewed by others
References
H. Savolainen, Rev. Environ. Health, 11, 167–173 (1996).
World Health Organization Guidelines for Drinking-Water Quality, 4th ed., Gutenberg, WHO Press (2011).
F. S. Wei, P. H. Qu, N. K. Shen, and F. Yin, Talanta, 28, 189–191 (1981).
X. K. Ping, Rock Miner. Anal., 2, 101–106 (1988).
S. L. C. Ferreira, A. C. S. Costa, and D. S. de Jesus, Talanta, 43, 1649–1656 (1996).
K. Sözgen and E. Tütem, Talanta, 62, 971–976 (2004).
S. J. Chen, X. S. Zhang, L. Y. Yu, L. Wang, and H. Li, Spectrochim. Acta, A, 88, 49–55 (2012).
Water and Wastewater Monitoring and Analysis Method, 4th ed., China Environmental Science Press, Beijing (2003).
Environmental Chemistry, Higher Education Press, Beijing (2003).
Complexation in Analytical Chemistry, Interscience Co., New York (2006).
The Analytical Uses of Ethylenediamine Tetraacetic Acid, Van Nostrand Co., New York (1965).
J. Z. Zhang, X. H. Fan, and L. H. Xue, Metall. Anal., 8, 74–78 (2011).
Environmental Chemical and Biological Effects of Trace Elements, China Environmental Science Press, Beijing (1992).
Z. Q. Zhou, Z. W. Yu, H. Y. Chen, and L. R. Chen, Chem. Res., 4, 9–12 (1997).
P. E. Mermet and J. M. Deruaz, J. Anal. At. Spectrom., 2, 61–65 (1994).
G. T. He, Y. P. Du, L. H. Ping, and L. Q. Ying, Chin. J. Spectrosc. Lab., 26, 1120–1125 (2009).
GB11910-89, National Standard of P. R. China (1989).
W. B. Jin and Q. Lu, Metall. Anal., 6, 76–77 (2007).
H. W. Ji, H. X. Cao, H. Z. Xin, and S. Li, J. Ocean Univ. China, 1, 25–30 (2010).
X. Li, Metall. Anal., 3, 71–74 (2007).
GB11912-89, National Standard of P. R. China (1989).
A. R. Khorrami and A. R. Fakhari, Talanta, 64, 13–17 (2004).
H. Y. Luo, W. H. Ruan, Y. G. Chen, Y. X. Mo, L. P. Zhu, Y. L. Wu, and J. L. Li, Mod. Food Sci. Technol., 12, 1527–1529 (2011).
Z. Q. Yang and A. X. Yu, Anal. Test Technol. Instrum., 2, 98–100 (2003).
S. L. C. Ferreira, C. F. Brito, A. F. Dantas, N. M. Araújo, and A. C. S. Costa, Talanta, 48, 1173–1177 (1999).
K. Chisato, U. Kan, H. Satsuki, D. Tomotaro, and K. Koichi, Bull Osaka Med. Coll., 1, 1–7 (2005).
M. Morfobos and A. Economou, Anal. Chim. Acta, 519, 57–64 (2004).
C. Kokkinos and A. Economou, Anal. Chim. Acta, 622, 111–118 (2008).
H. Wu, Z. P. Wang, and G. S. Chen, Metall. Anal., 6, 15–17 (1999).
Y. Ma, G. L. Fan, and C. R. Gong, Mod . Sci. Instrum., 5, 116–118 (2007).
Catalytic Kinetic Analysis and Its Application, Jiangxi College Publ. House, Nanchang (1991).
O. D. Renedo, M. A. A. Lomillo, and M. J. A. Martinez, Anal. Chim. Acta, 521, 215–221 (2004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Abstract of article is published in Zhurnal Prikladnoi Spektroskopii, Vol. 82, No. 5, p. 807, September–October, 2015.
Rights and permissions
About this article
Cite this article
Zhao, Z.X., Zhang, C.X., Li, N. et al. Determination of Trace Nickel in Natural Water by Flow Injection Analysis with Cetrimonium Bromide as Sensitizer. J Appl Spectrosc 82, 882–887 (2015). https://doi.org/10.1007/s10812-015-0198-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-015-0198-5