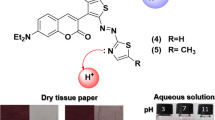
A multifunctional water-soluble azo dye with the D-π-A conjugated structure, 4-(4-hydroxy-1-naphthylazo) benzoic acid ( HNBA), was designed and synthesized using 1-naphanol as the electron donator, benzoic acid as the electron acceptor, and −N=N− as the bridging group. After its structure was characterized by FTIR, 1H NMR, and element analysis, the UV-Vis absorption spectral performance of the target dye was studied in detail. The results showed that the dye, combining hydroxyl group, azo group, and carboxyl group, possessed excellent absorption spectral properties (ε = 1.2·104 l·mol−1·cm−1) changing with pH and solvents. In particular, in polar and protonic water, it had excellent optical response to some metal ions, i.e., Fe3+ and Pb2+, which might make it a latent colorimetric sensor for detecting heavy metal ions.
Similar content being viewed by others
References
G. Leng, L. Feng, S. Li, S. Qian, and D. Dan, Environ. Forens ics, 14, 9–15 (2013).
M. Legrand, R. Lam, M. Jensen-Fontaine, E. Salin, and H. Chan, J. Anal. At. Spectrom., 19, 1287–1288 (2004).
H. Kodamatani, A. Matsuyama, K. Saito, Y. Kono, R. Kanzaki, and T. Tomiyasu, Anal. Sci., 28, 959–965 (2012).
T. Oshea and S. Lunte, Anal. Chem., 65, 247–250 (1993).
W. Lu, X. Qin, S. Liu, G. Chang, Y. Zhang, and Y. Luo, Anal. Chem., 84, No. 12, 5351–5357 (2012).
Z. Yan, H. Xue, K. Berning, Y. Lam, and C. Lee, ACS App l. Mater. Interfaces, 6, No. 24, 22761–22768 (2014).
Z. Yan, M. Yuen, L. Hu, P. Sun, and C. Lee, RSC Adv., 4, 48373–48388 (2014).
L. Hu, L. Nie, G. Xu, H. Shi, X. Xu, X. Zhang, and Z. Yan, RSC Adv., 4, 19370–19374 (2014).
L. Hu, Z. Yan, and H. Xu, RSC Adv., 3, 7667–7676 (2013).
Z. Yan, H. Xu, S. Guang, X. Zhao, W. Fan, and X. Liu, Adv. Funct. Mater., 22, 345–352 (2012).
D. Avnir, S. Braun, and M. Ottolenghi, ACS Symp. Ser., 499, 384–404 (1992).
S. Basu, Ind. Eng. Chem. Prod. Res. Dev., 23, 183–186 (1984).
13 . Z. Yan, S. Guang, H. Xu, and X. Liu, Dyes Pigm., 99, 720–726 (2013).
B. Kolli, S. Pandey , S. Mishra, T. Kanai, M. Joshi, R. Mohan, T. Dhami, L. Kukreja, and A. Samui, J. Polym. Sci., Part A: Polym. Chem., 51, 4317–4324 (2013).
Y. Zhang, J. Martinez-Perdiguero, U. Baumeister, C. Walker, J. Etxebarria, M. Prehm, J. Ortega, C. Tschierske, M. O’Callaghan, A. Harant, and M. Handschy, J. Am. Chem. Soc., 131, 18386–18392 (2009).
F. Li, Y. Zhang, C. Wu, Z. Lin, B. Zhang, and T. Guo, Vacuum, 86, 1895–1897 (2012).
T. Kuo, S. Lin, Y. Hung, J. Horng, and M. Houng, IEEE Photonics Technol. Lett., 23, 362–364 (2011).
S. Chen and C. Yu, Microsc. Res. Technol., 73, 202–205 (2010).
Z. Yan, L. Hu, L. Nie, and H. Lv, Spectrochim. Acta, A, 79, 661–665 (2011).
L. Hu, Y. Zhang, L. Nie, C. Xie, and Z. Yan, Spectrochim. Acta, A, 104, 87–91 (2013).
F. Gou, X. Jiang, B. Li, H. Jing, and Z. Zhu, ACS Appl. Mater. Interfaces, 5, 12631–12637 (2013).
H. Singh, J. Sindhu, J. Khurana, C. Sharma, and K. Aneja, RSC Adv., 4, 5915–5926 (2014).
E. Rufchahi, H. Pouramir, M. Yazdanbakhsh, H. Yousefi , M. Bagheri, and M. Rassa, Chin. Chem. Lett., 24, 425–428 (2013).
M. Jeanmougin, D. Bonvalet, J. Civatte, A. Ramelet, and C. Vilmer, Ann. Dermatol. Vener., 111, 437–444 (1984).
H. El-Desoky, M. Ghoneim, R. El-Sheikh, and N. Zidan, J. Hazard. Mater., 175, 858–865 (2010).
C. Carliell , S. Barclay, C. Shaw, A. Wheatley, and C. Buckley, Environ. Technol., 19, 1133–1137 (1998).
J. Yun and H. Choi, Talanta, 52, 893–902 (2000).
R. Ebdelli, A. Rouis, R. Mlika, I. Bonnamour, H. Ouada, and J. Davenas, J. Mol. Struct., 1006, 210–215 (2011).
Z. Yan, Y. Chen, S. Guang, H. Xu, and L. Li, Polym. Sci. Ser. B, 53, 535–539 (2011).
Y. Shigeyuki, H. Yutaka, H. Masahiko, N. Hiroyuki, S. Yoshiaki, and A. Ajayaghosh, Org. Lett., 9, 1999–2002 (2007).
A. Ajayaghosh, P. Chithra, R. Varghese, and K. Divya, C hem. Commun., 969–971 (2008).
Gaussian 03, revisio n A.1, Gaussian, Inc., Pittsburgh, PA (2004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Zhurnal Prikladnoi Spektroskopii, Vol. 82, No. 3, pp. 436–440, May–June, 2015.
Rights and permissions
About this article
Cite this article
Hu, L., Lv, H., Xie, C.G. et al. Preparation and Absorption Spectral Property of a Multifunctional Water-Soluble Azo Compound with D-π-A Structure, 4-(4- Hydroxy-1-Naphthylazo)Benzoic Acid. J Appl Spectrosc 82, 445–449 (2015). https://doi.org/10.1007/s10812-015-0127-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-015-0127-7