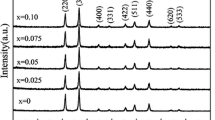
Single-phase Nd-doped YVO4 nanopowders were synthesized by a chemical method. The powders were heated at 1200°C for 1 h to obtain good crystallinity with better luminescence. Annealing temperature affects the crystal structure of the nanopowders. X-ray diffraction (XRD) revealed that a preferred crystallographic orientation is [200]. Measurements showed that the nanoparticles had an average size of 62.5 nm. A scanning electron microscope (SEM) image of the nanopowders indicated the formation of uniform and nearly spherical Nd:YVO4 particles. The transmittance spectrum of the nanopowders showed a broad absorption band around 808 nm. Photoluminescence studies on the annealed powders revealed their luminescence properties.
Similar content being viewed by others
References
J. R. O’Connor, Appl. Phys. Lett., 9, No. 11, 407–410 (1966).
A. W. Tucher, M. Birnbaun, C. L. Fincher, L. G. Deshazer, J. Appl. Phys., 47, No. 1, 232–235 (1976).
A. W. Tucher, M. Birebaun, C. L. Fincher, J. W. Erler, J. Appl. Phys., 48, No. 12, 4907–4911 (1977).
R. A. Fields, M. Birnbaun, C. L. Fincher, Appl. Phys. Lett., 51, No. 23, 1885–1887 (1987).
G. C. Bowkett, Nd:YVO4 Microchip Lasers and Amplifiers, PhD thesis, Victoria Univ. of Technol. (1999).
M. Yu, J. Lin, Z. Wang, J. Fu, S. Wang, H. J. Zhang, Y. C. Han, Chem. Mater., 14, 2224 (2002).
S. Ekambaram, K. C. Patil, J. Alloys Compd., 217, No. 1, 104–107 (1995).
L. D. Sun, Y. X. Zhang, J. Zhang, C. H. Yan, C. S. Liao, Y. Q. Lu, Solid State Commun., 124, No. 1, 35–38 (2002).
H. S. Lai, B. J. Chen, W. Xu, X. J. Wang, Y. M. Yang, Q. Y. Meng, J. Alloys Compd., 395, No. 1, 181–184 (2005).
H. Arnaud, G. Thierry, Chem. Mater., 12, 1090 (2002).
H. Wu, H. Xu, Q. Su, T. Chen, M. Wu, J. Mater. Chem., 13, No. 5, 1223–1228 (2003).
A. Huignard, T. Gacoin, J. P. Boilot, Chem. Mater., 12, No. 4, 1090–1094 (2000).
L. M. Chen, Y. N. Liu, K. L. Huang, Mater. Res. Bull., 41, No. 1, 158–166 (2006).
R. C. Ropp, B. Carroll, J. Inorg. Nucl. Chem., 39, No. 8, 1303–1307 (1977).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Zhurnal Prikladnoi Spektroskopii, Vol. 80, No. 6, pp. 961–963, November–December, 2013.
Rights and permissions
About this article
Cite this article
Maleki, M.H., Alhooie, S., Dizaji, H.R. et al. Synthesis and Physical Properties of Nd:YVO4 Nanopowders prepared by a Chemical Method. J Appl Spectrosc 80, 954–956 (2014). https://doi.org/10.1007/s10812-014-9872-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-014-9872-2