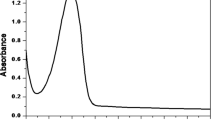
A spectrophotometric method is proposed for the determination of norepinephrine (NE) and its bitartrate salts. The method was based on the development of a red color (λmax = 495 nm) with sodium iodate in aqueous alcoholic medium at pH 5. The color was stable for at least 4 hrs. The molar reacting ratio of NE to sodium iodate was 1:4. A linear relationship was obtained between the absorption intensity and NE concentration in the range of 3.384–37.224 μg/ml with detection limit of 0.067 μg/ml and correlation coefficient of 0.9972. The present work facilitated the determination of the three acidity constants, 7.564 ± 0.02, 9.036 ± 0.034, and 10.761 ± 0.023. The reaction mechanism was also described. The proposed method was successfully applied for the determination of NE in pharmaceutical formulations. Results for analysis of bulk drugs and injections agree with those of official methods.
Similar content being viewed by others
References
B. G. Katzung, Basic & Clinical Pharmacology, 6th ed., Appleton & Lange, Connecticut, (1995).
C. Martin, L. Papazian, G. Perrin, and F. Gouin, Chest, 103, 1826 (1993).
P. Desjars, M. Pinaud, G. Potel, F. Tasseau, and M. D. Touze, Crit. Care Med, 15, 134 (1987).
J. L. Moran, M. O. Fathartaigh, A. R. Peisach, M. J. Chapman, and P. Leppard, Crit. Care Med., 21, 70 (1993).
H. Jeong, H. Kim, and S. Jeon, Microchem. J., 78, 181 (2004).
C. Bian, Q. Zeng, H. Xiong, X. Zhang, and S. Wang, Bioelectrochemistry, 79, 1 (2010).
Y. Li, X. Huang, Y. Chen, L. Wang, and X. Lin, Microchim. Acta, 164, 107 (2009).
M. Mazloum-Ardakani, H. Rajabi, H. Beitollahi, B. B. F. Mirjalili, A. Akbari, and N. Taghavinia, Int. J. Electrochem. Sci, 5, 147 (2010).
T. Yoshitake, K. Fujino, J. Kehr, J. Ishida, H. Nohta, and M. Yamaguchi, Anal. Biochem., 312, 125 (2003).
M. A. Fotopoulou and P. C. Ioannou, Anal. Chim. Acta, 462, 179 (2002).
Z. D. Peterson, D. C. Collins, C. R. Bowerbank, M. L. Lee, and S. W. Graves, J . Chromatogr. B, 776, 221 (2002).
D. L. Kuhlenbeck, T. P. O’Neill, C. E. Mack, S. H. Hoke, and K. R. Wehmeyer, J . Chromatogr. B, 738, 319 (2000).
G. H. Ragab, H. Nohta, and K. Zaitsu, Anal. Chim. Acta, 403, 155 (2000).
G. H. Ragab, H. Nohta, M. Kai, Y. Ohkura, and K. Zaitsu, J . Pharm. Biomed. Anal., 13, 645 (1995).
J. J. B. Nevado, J. M. L. Gallego, and P. B. Laguna, Anal. Chim. Acta, 300, 293 (1995).
J. R. Doty, Anal. Chem., 20, 1166 (1948).
P. Nagaraja, K. C. S. Murthy, K. S. Rangappa, and N. M. M. Gowda, Talanta, 46, 39 (1998).
M. A. Korany, A. M. Wahbi, and M. H. Abdel-Hady, J. Pharm. Biomed. Anal., 2, 537 (1994).
A. G. Davidson, J. Pharm. Biomed. Anal., 2, 45 (1984).
R. T. Sane, P. M. Deshpande, C. L. Sawant, S. M. Dolas, V. G. Nayak, and S. S. Zarapkar, Indian Drugs, 24, 199 (1987).
M. H. Sorouraddin, J. L. Manzoori, E. Kargarzadeh, and A. M. H. Shabani, J. Pharm. Biomed. Anal., 18, 877 (1998).
M. Zhu, X. Huang, and H. Shen, Anal. Chim. Acta, 357, 261 (1997).
F. B. Salem, Talanta, 34, 810 (1987).
M. E. El-Kommos, F. A. Mohamed, and A. S. Khedr, J. Assoc. Off. Anal. Chem., 73, 516 (1990).
J. Yang, G. Zhang, X. Wu, F. Huang, C. Lin, X. Cao, L. Sun, and Y. Ding, Anal. Chim. Acta, 363, 105 (1998).
H. Y. Wang, Q. S. Hui, L. X. Xu, J. G. Jiang, and Y. Sun, Anal. Chim. Acta, 497, 93 (2003).
Y. Liu, J. Yang, X. Wu, and L. Li, J. Fluoresc., 13, 123 (2003).
M. M. Karim, S. M. Alam, and S. H. Lee, J. Fluoresc., 17, 427 (2007).
A. E. Sanchez-Rivera, S. Corona-Avendano, G. Alacorn-Angeles, A. Rojas-Hernandez, M. T. Ramirez-Silva, and M. A. Romero-Romo, Spectrochim. Acta, A, 59, 3193 (2003).
J. C. Miller and J. N. Miller, Statistics for Analytical Chemistry, Wiley-VCH, New York, (1984).
R. A. Heacock, in Advances in Heterocyclic Chemistry, Eds. A. R. Katritzky, A. J. Boulton, and J. M. Lagowski Academic Press, New York, (1965), p. 17.
R. A. Heacock and W. S. Powell, in Progress in Medicinal Chemistry, Eds. G. P. Ellis, G. B. West, North-Holland, Amsterdam, (1973), p. 291.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Zhurnal Prikladnoi Spektroskopii, Vol. 80, No. 2, pp. 226–272, March–April, 2013.
Rights and permissions
About this article
Cite this article
Hashem, E.Y., Youssef, A.K. Spectrophotometric determination of norepinephrine with sodium iodate and determination of its acidity constants. J Appl Spectrosc 80, 258–264 (2013). https://doi.org/10.1007/s10812-013-9755-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-013-9755-y