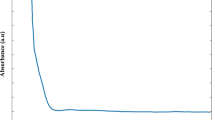
Optical properties (photoluminescence and absorption) of Eu(bta)3(B) n (B = H2O or 1,10-phenanthroline) polycrystalline powders and fluoroacrylate polymers (FAPs) impregnated with these compounds using supercritical CO2 (SC CO2) were investigated. It was established that impregnation of Eu(bta)3phen into the FAPs using an SC CO2 solution was difficult to achieve. The type of B (ancillary ligand) and the polymer matrix were shown to influence the temperature quenching of photoluminescence of Eu3+ ions in the range 25–100°C. A comparative analysis of quantum yields (λex = 300 and 380 nm) and photoluminescence decay times (λex = 337.1 nm) for Eu(bta)3B n and for Eu(bta)3B n -doped FAPs was performed.
Similar content being viewed by others
References
S. V. Eliseeva and J.-C. G. Bunzli, Chem. Soc. Rev., 39, 189–227 (2010).
A. I. Cooper, J. Mater. Chem., 10, 207–234 (2000).
V. I. Gerasimova, Yu. S. Zavorotny, A. O. Rybaltovskii, A. Yu. Chebrova, N. L. Semenova, D. A. Lemenovskii, and Yu. L. Slovohotov, J. Luminesc., 129, 1115–1119 (2009).
V. I. Gerasimova, Yu. S. Zavorotnyi, A. O. Rybaltovskii, A. A. Antoshkov, V. I. Sokolov, E. V. Troitskaya, and V. N. Bagratashvili, Sverkhkrit. Flyuidy: Teor. Prakt., 5, 56–69 (2010).
G. F. Da Sa, O. L. Malta, C. De Mello Donega, A. M. Simas, R. L. Longo, P. A. Santa-Cruz, and E. F. Da Silva, Jr., Coord. Chem. Rev., 196, 165–195 (2000).
M. T. Berry, P. S. May, and H. Xu, J. Phys. Chem., 100, 9216–9222 (1996).
J. Feng, H.-J. Zhang, S.-Y. Song, Z.-F. Li, L.-N. Sun, Y. Xing, and X.-M. Guo, J. Luminesc., 128, 1957–1964 (2008).
E. V. Triotskaya, S. I. Molchanova, and V. I. Sokolov, in: Proceedings of the XIIth Int. Sci. Conf. "Physicochemical Processes for Selection of Atoms and Molecules in Laser, Plasma, and Nanotechnology" [in Russian], March 31–April 4, 2008, Zvenigorod (2008), 215–220.
M. O. Gallyamov, R. A. Vinokur, L. N. Nikitin, E. E. Said-Galiyev, A. R. Khokhlov, and K. Schaumburg, Polym. Sci., Ser. A, 44, 581–592 (2002).
S. T. Frey and W. W. Horrocks, Inorg. Chim. Acta, 229, 383–390 (1995).
A. A. Sviridova, A. B. Solov?eva, A. O. Rybaltovskii, V. A. Timofeeva, A. V. Krivandin, O. V. Shatalova, N. N. Glagolev, T. S. Zarkhina, and V. N. Bagratashvili, Sverkhkrit. Flyuidy: Teor. Prakt., 1, 13–22 (2006).
A. O. Rybaltovskii, V. I. Gerasimova, Yu. S. Zavorotnyi, and A. Yu. Chebrova, Zh. Prikl. Spektrosk., 76, No. 1, 104–111 (2009).
Zh. V. Dobrokhotova, I. G. Fomina, G. G. Aleksandrov, Yu. A. Velikodnyi, V. N. Ikorskii, A. S. Bogomyakov, L. N. Puntus, V. M. Novotortsev, and I. L. Eremenko, Zh. Neorg. Khim., 54, 727–744 (2009).
P. Dao and A. J. Twarowski, J. Chem. Phys., 85, 6823–6827 (1986).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Prikladnoi Spektroskopii, Vol. 79, No. 2, pp. 220–227, March–April, 2012.
Rights and permissions
About this article
Cite this article
Gerasimova, V.I., Antoshkov, A.A., Zavorotny, Y.S. et al. Photoluminescence of fluoroacrylate polymers impregnated with Eu(bta)3 using supercritical CO2 . J Appl Spectrosc 79, 203–210 (2012). https://doi.org/10.1007/s10812-012-9584-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-012-9584-4