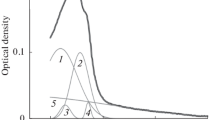
The application of a maximum entropy method (MEM) for analysis of time-resolved fluorescence data is discussed. A developed version of MEM has been tested using simulated kinetic data. Based on computed results, practical criteria have been established to determine whether the lifetime distribution of emitting centers is described by a discrete spectrum (a set of two or three exponentials) or by a continuous one (mono- or bimodal distribution of exponentials). The proposed method has been used to analyze the fluorescence decay kinetics of thioflavin T (ThT) intercalated into amyloid fibrils. The presence of two peaks in the lifetime distribution of emitting centers has been explained by the existence in fibrils of two types of binding centers substantially differing in microenvironment rigidity. This suggestion is supported by the results of fluorescence quenching of intercalated ThT with the quencher KI.
Similar content being viewed by others
References
D. V. O’Connor and D. Phillips, Time-correlated Single Photon Counting, Academic Press, New York (1984).
W. R. Ware, in: Time-resolved Fluorescence Spectroscopy in Biochemistry and Biology, R. B. Cundall, ed., Plenum Press, New York (1983), p. 23.
A. N. Tikhonov and V. Ya. Arsenin, Methods for Solving Ill-Conditioned Problems [in Russian], Nauka, Moscow (1974).
L. Dobrynski and A. Holas, Nucl. Instrum. Methods Phys. Res., Sect. A, 383, 589 (1996).
R. D. Dyson and I. Isenberg, Biochemistry, 10, 3233–3241 (1971).
A. Grinvald and I. Z. Steinberg, Anal. Biochem., 59, 583–598 (1974).
S. K. Basharin, G. A. Gachko, L. N. Kivach, S. A. Maskevich, A. A. Maskevich, and V. R. Udovydchenko, Zh. Prikl. Spektrosk., 52, No. 1, 48–52 (1990).
H. P. Good, A. J. Kaller, and U. P. Wild, J. Phys. Chem., No. 22, 5435–5441 (1984).
J. Sopkova, J. Gallay, M. Vincent, P. Pancoska, and A. Lewit-Bentley, Biochemistry, 33, 4490–4499 (1994).
J. Sopkova, M. Vincent, M. Takahashi, A. Lewit-Bentley, and J. Gallay, Biochemistry, 37, 11962–11970 (1998).
A. A. Maskevich, S. K. Basharin, G. A. Gachko, L. N. Kivach, and S. A. Maskevich, Zh. Prikl. Spektrosk., 53, No. 4, 557–563 (1990).
N. Rouviere, M. Vincent, C. T. Craescu, and J. Gallay, Biochemistry, 36, 7339–7352 (1997).
E. Bismuto, G. Irace, S. D’Auria, M. Rossi, and R. Nucci, Eur. J. Biochem., 244, 53–58 (1997).
J. K. A. Kamal and D. V. Behere, Biochem. Biophys. Res. Commun., 289, 427–433 (2001).
J. G. McWhirter and E. R. Pike, J. Phys. A: Math. Gen., 11, 1729–1745 (1978).
A. Siemiarczuk, B. D. Wagner, and W. R. Ware, J. Phys. Chem., 94, 1661–1666 (1990).
J.-C. Brochon, Methods Enzymol., 240, 262–311 (1994).
D. R. James and W. R. Ware, Chem. Phys. Lett., 120, 455–459 (1985).
G. Landl, T. Langthaler, H. W. Engl, and H. F. Kauffman, J. Comput. Phys., 95, 1–28 (1991).
M. A. Noginov, S. E. Sverchkov, and Yu. E. Sverchkov, Several Inverse Problems of Kinetic Spectroscopy of Impurity Solids [in Russian], Preprint No. 62, IOP, AS USSR, Moscow (1988).
E. P. Petrov, J. V. Kruchenok, and A. N. Rubinov, J. Fluoresc., 9, 111–121 (1999).
A. K. Livesey and J.-C. Brochon, Biophys. J., 52, 693–706 (1987).
P. J. Steinbach, R. Ionescu, and C. R. Matthews, Biophys. J., 82, 2244–2255 (2002).
R. Willingale, Mon. Not. R. Astron. Soc., 194, 359–364 (1981).
Z. Ablonczy, A. Lukacs, and E. Papp, Biophys. Chem., 104, 249–258 (2003).
R. Swaminathan, G. Krishnamoorthy, and N. Periasamy, Biophys. J., 67, 2013–2023 (1994).
T. N. Anand Kumar, J. F. Leyun, C. Zhu, A. A. Demidov, and P. M. Champion, J. Phys. Chem. B, 105, 7847–7856 (2001).
S. Sibisi, J. Skilling, R. G. Brereton, E. D. Lane, and J. Staunton, Nature, 311, 446–447 (1984).
T. Uchiyama, H. Minamitani, and M. Sakata, Jpn. J. Appl. Phys., 29, 212–218 (1990).
J. Skilling, ed., in: Maximum Entropy and Bayesian Methods, Kluwer Academic, Norwell (1989), pp. 45–52.
A. A. Maskevich, V. I. Stsiapura, and P. T. Balinski, in: Articles from the International Scientific Conference "Molecular Membranes and Cellular Bases of Biosystem Functioning" and Sixth Conference of the Belarusian Society for Education of Photobiologists and Biophysicists [in Russian], Minsk, October 6–8, 2004, RB President Center for Academic Management (2004), pp. 156–158.
D. W. Marquardt, J. Soc. Ind. Appl. Math., 11, 431–441 (1963).
E. S. Voropai, M. P. Samtsov, K. N. Kaplevskii, A. A. Maskevich, V. I. Stsiapura, O. I. Povarova, I. M. Kuznetsova, K. K. Turoverov, A. L. Fink, and V. N. Uverskii, Zh. Prikl. Spektrosk., 70, No. 6, 767–773 (2003).
A. A. Maskevich, V. I. Stsiapura, V. A. Kuzmitsky, I. M. Kuznetsova, O. I. Povarova, V. N. Uversky, and K. K. Turoverov, J. Proteome Res., 6, 1392–1401 (2007).
V. I. Stsiapura, A. A. Maskevich, V. A. Kuzmitsky, K. K. Turoverov, and I. M. Kuznetsova, J. Phys. Chem. A, 111, 4829–4835 (2007).
V. I. Stsiapura, A. A. Maskevich, V. A. Kuzmitsky, V. N. Uversky, I. M. Kuznetsova, and K. K. Turoverov, J. Phys. Chem. B, 112, 15893–15902 (2008).
J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Plenum Press, New York (1983).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Prikladnoi Spektroskopii, Vol. 77, No. 2, pp. 209–217, March–April, 2010.
Rights and permissions
About this article
Cite this article
Maskevich, A.A., Stsiapura, V.I. & Balinski, P.T. Analysis of fluorescence decay kinetics of thioflavin t by a maximum entropy method. J Appl Spectrosc 77, 194–201 (2010). https://doi.org/10.1007/s10812-010-9314-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-010-9314-8