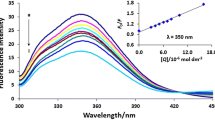
We have used fluorescence spectroscopy methods to show that imidacloprid and its structural analogs form complexes with human serum albumin (HSA). The nature of the spectral changes in the ligand×protein systems and the calculated complexation parameters suggest that these low molecular weight compounds mainly bind to a specific section of the protein molecule, near the tryptophan residue in the 214 position of the polypeptide chain. We have found that the association constants are on the order of 104 M−1, and the affinity of the ligands for HSA varies in the series 6-chloronicotinic acid > 6-methoxynicotinic acid = imidacloprid > the keto analog of imidacloprid. The major contribution to the complexation energy probably comes from hydrophobic interaction forces with participation of the aromatic pyridine ring of the ligands, while additional enhancement of ligand-protein affinity can be provided by the nitroimine group of imidacloprid.
Similar content being viewed by others
References
K. Matsuda, S. D. Buckingham, D. Kleier, J. J. Rauh, M. Grauso, and D. B. Sattelle, Trends Pharmacol. Sci., 22, 573–580 (2001).
M. Tomizawa and J. E. Casida, Ann. Rev. Pharmacol. Toxicol., 45, 247–268 (2005).
I. Yamamoto, M. Tomizawa, T. Saito, T. Miyamoto, E. C. Walcott, and K. Sumikawa, Arch. Insect. Biochem. Physiol., 37, 24–32 (1998).
K. Matsuda, M. Shimomura, M. Ihara, M. Akamatsu, and D. B. Sattelle, Biosci. Biotechnol. Biochem., 69, 1442–1452 (2005).
M. Tomizawa, T. T. Talley, D. Maltby, K. A. Durkin, K. F. Medzihradszky, A. L. Burlingame, P. Taylor, and J. E. Casida, Proc. Natl. Acad. Sci. USA, 104, 9075–9080 (2007).
Y. Wang, J. Cheng, X. Qian, and Z. Li, Bioorg. Med. Chem., 15, 2624–2630 (2007).
F. A. De Wolf and G. M. Brett, Pharmacol. Rev., 52, 207–236 (2000).
C. M. Mendel, Endocrinol. Rev., 10, 232–274 (1989).
V. D. Luk'yanchuk and A. I. Luik, Zh. Klin. i Éksp. Meditsiny, 23, 33–37 (1983).
T. Cserhati and E. Forgacs, J. Chromatogr. A, 699, 285–290 (1995).
M. Gulden, S. Tahan, and H. Seibert, Toxicology, 175, 201–213 (2002).
D. Silva, C. M. Cortez, J. Cunha-Bastos, and S. R. Louro, Toxicol. Lett., 147, 53–61 (2004).
E. S. Peeples, L. M. Schopfer, E. G. Duysen, R. Spaulding, T. Voelker, C. M. Thompson, and O. Lockridge, Toxicol. Sci., 83, 303–312 (2005).
I. Mukherjee, Pest. Management Sci., 56, 932–936 (2000).
N. V. Kovganko, Yu. G. Chernov, V. L. Survilo, and Zh. N. Kashkan, “Method for obtaining imidacloprid,” Belarus Patent No. 8491, IPC7 C07D401/06 (2004).
M.-X. Xie, X.-Y. Xu, and Y.-D. Wang, Biophys. Biochim. Acta, 1724, 215–224 (2005).
A. I. Luik and V. D. Luk'yanchuk, Serum Albumin and Biotransport of Toxins [in Russian], Meditsina, Moscow (1984), pp. 45–65.
J. R. Lakowicz, Principles of Fluorescence Spectroscopy [Russian translation from English; M. G. Kuz'min, ed.], Mir, Moscow (1986).
S. Sugio, A. Kashima, S. Mochizuki, M. Noda, and K. Kobayashi, Protein Eng., 12, 439–446 (1999).
G. Sudlow, D. J. Birkett, and D. N. Wade, Mol. Pharmacol., 11, 824–832 (1975).
Y. V. Il'ichev, J. L. Perry, and J. D. Simon, J. Phys. Chem. B, 106, 460–465 (2002).
A. Bertuzzi, G. Mingrone, A. Gandolfi, A. V. Greco, S. Ringoir, and R. Vanholder, Clin. Chim. Acta, 265, 183–192 (1997).
H. Athar, N. Ahmad, S. Tayyab, and M. A. Qasim, Int. J. Biol. Macromol., 25, 353–358 (1999).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Prikladnoi Spektroskopii, Vol. 75, No. 6, pp. 859–866, November–December, 2008.
Rights and permissions
About this article
Cite this article
Mikhailopulo, K.I., Serchenya, T.S., Kiseleva, E.P. et al. Interaction of molecules of the neonicotinoid imidacloprid and its structural analogs with human serum albumin. J Appl Spectrosc 75, 857–863 (2008). https://doi.org/10.1007/s10812-009-9120-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-009-9120-3