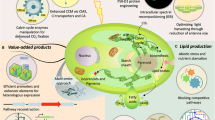
Abstract
Eukaryotic microalgae are important primary producers in nature that play an important role in the energy cycle of nature. The eukaryotic microalgae are also regarded as potential bioactive substance producers. In recent years, with the aid of genetic engineering, eukaryotic microalgae have found a wider range of potential applications in medicine, food, health products, cosmetics, and environmental protection. This article reviews the state of the art of microalga genetic engineering from the aspects of gene transfer system, gene editing technology, and screening method, with examples of the enhancement of lipids, carotenoids, polysaccharide, and functional proteins. Potential application scenarios of microalga genetic engineering and their products in the field of food and medicine are also highlighted. Furthermore, strategies for protein expression optimization in eukaryotic microalgae are reviewed. The existing shortcomings of eukaryotic microalga genetic engineering are also analyzed and highlighted.



Similar content being viewed by others
References
Ahmad I, Sharma AK, Daniell H, Kumar S (2015) Altered lipid composition and enhanced lipid production in green microalga by introduction of brassica diacylglycerol acyltransferase 2. Plant Biotechnol J 13:540–550
Aikawa S, Ho SH, Nakanishi A, Chang JS, Hasunuma T, Kondo A (2015) Improving polyglucan production in cyanobacteria and microalgae via cultivation design and metabolic engineering. Biotechnol J 10:886–898
Ajjawi I, Verruto J, Aqui M, Soriaga LB, Coppersmith J, Kwok K, Peach L, Orchard E, Kalb R, Xu W, Carlson TJ, Francis K, Konigsfeld K, Bartalis J, Schultz A, Lambert W, Schwartz AS, Brown R, Moellering ER (2017) Lipid production in Nannochloropsis gaditana is doubled by decreasing expression of a single transcriptional regulator. Nat Biotechnol 35:647–652
Allen GC, Spiker S, Thompson WF (2000) Use of matrix attachment regions (MARs) to minimize transgene silencing. Plant Mol Biol 43:361–376
Angstenberger M, de Signori F, Vecchi V, Dall'Osto L, Bassi R (2020) Cell synchronization enhances nuclear transformation and genome editing via Cas9 enabling homologous recombination in Chlamydomonas reinhardtii. ACS Synth Biol 9:2840–2850
Anila N, Chandrashekar A, Ravishankar GA, Sarada R (2011) Establishment of Agrobacterium tumefaciens-mediated genetic transformation in Dunaliella bardawil. Eur J Phycol 46:36–44
Anila N, Simon DP, Chandrashekar A, Ravishankar GA, Sarada R (2016) Metabolic engineering of Dunaliella salina for production of ketocarotenoids. Photosynth Res 127:321–333
Arad SM, Levy-Ontman O (2010) Red microalgal cell-wall polysaccharides: biotechnological aspects. Curr Opin Biotechnol 21:358–364
Baek K, Kim DH, Jeong J, Sim SJ, Melis A, Kim JS, Jin E, Bae S (2016) DNA-free two-gene knockout in Chlamydomonas reinhardtii via CRISPR-Cas9 ribonucleoproteins. Sci Rep 6:30620
Baek K, Yu J, Jeong J, Sim SJ, Bae S, Jin ES (2018) Photoautotrophic production of macular pigment in a Chlamydomonas reinhardtii strain generated by using DNA-free CRISPR-Cas9 RNP-mediated mutagenesis. Biotechnol Bioeng 115:719–728
Baier T, Wichmann J, Kruse O, Lauersen KJ (2018) Intron-containing algal transgenes mediate efficient recombinant gene expression in the green microalga Chlamydomonas reinhardtii. Nucleic Acids Res 46:6909–6919
Baier T, Jacobebbinghaus N, Einhaus A, Lauersen KJ, Kruse O (2020) Introns mediate post-transcriptional enhancement of nuclear gene expression in the green microalga Chlamydomonas reinhardtii. PLoS Genet 16. https://doi.org/10.1371/JOURNAL.PGEN.1008944
Barahimipour R, Neupert J, Bock R (2016) Efficient expression of nuclear transgenes in the green alga Chlamydomonas: synthesis of an HIV antigen and development of a new selectable marker. Plant Mol Biol 90:403–418
Barka F, Angstenberger M, Ahrendt T, Lorenzen W, Bode HB, Büchel C (2016) Identification of a triacylglycerol lipase in the diatom Phaeodactylum tricornutum. Biochim Biophys Acta 1861:239–248
Bashir KMI, Kim M-S, Stahl U, Cho M-G (2018) Agrobacterium-mediated genetic transformation of Dictyosphaerium pulchellum for the expression of erythropoietin. J Appl Phycol 30:3503–3518
Bertalan I, Munder MC, Weiß C, Kopf J, Fischer D, Johanningmeier U (2015) A rapid, modular and marker-free chloroplast expression system for the green alga Chlamydomonas reinhardtii. J Biotechnol 195:60–66
Bhattacharyya S, Pattanaik S, Maiti IB (2003) Intron-mediated enhancement of gene expression in transgenic plants using chimeric constructs composed of the Peanut chlorotic streak virus (PClSV) promoter–leader and the antisense orientation of PClSV ORF VII (p7R). Planta 218:115–124
Blanc G, Duncan GA, Agarkova I, Borodovsky M, Gurnon J, Kuo A, Lindquist E, Lucas S, Pangilinan J, Polle J, Salamov A, Terry A, Yamada T, Dunigan DD, Grigoriev IV, Claverie J-M, Etten JLV (2010) The Chlorella variabilis NC64A genome reveals adaptation to photosymbiosis, coevolution with viruses, and cryptic sex. Plant Cell 22:2943–2955
Blanc G, Agarkova IV, Grimwood J, Kuo A, Brueggeman AJ, Dunigan D, Gurnon J, Ladunga I, Lindquist E, Lucas S, Pangilinan J, Pröschold T, Salamov A, Schmutz J, Weeks DP, Yamada T, Lomsadze A, Borodovsky M, Claverie J-M, Grigoriev IV, Etten JLV (2012) The genome of the polar eukaryotic microalga Coccomyxa subellipsoidea reveals traits of cold adaptation. Genome Biol 13:1–12
Borovsky D, Sterner A, Powell CA (2016) Cloning and expressing trypsin modulating oostatic factor in Chlorella desiccata to control mosquito larvae. Arch Insect Biochem Physiol 91:17–36
Boynton JE, Gillham NW, Harris EH, Hosler JP, Johnson AM, Jones AR, Randolph-Anderson BL, Robertson D, Klein TM, Shark KB (1988) Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science 240:1534–1538
Breuer G, Lamers PP, Martens DE, Draaisma RB, Wijffels RH (2012) The impact of nitrogen starvation on the dynamics of triacylglycerol accumulation in nine microalgae strains. Bioresour Technol 124:217–226
Brown LE, Sprecher SL, Keller LR (1991) Introduction of exogenous DNA into Chlamydomonas reinhardtii by electroporation. Mol Cell Biol 11:2328–2332
Bruggeman AJ, Kuehler D, Weeks DP (2014) Evaluation of three herbicide resistance genes for use in genetic transformations and for potential crop protection in algae production. Plant Biotechnol J 12:894–902
Busi MV, Barchiesi J, Martín M, Gomez-Casati DF (2014) Starch metabolism in green algae. Starch-Starke 66:28–40
Castejon N, Senorans FJ (2020) Enzymatic modification to produce health-promoting lipids from fish oil, algae and other new omega-3 sources: a review. Nat Biotechnol 57:45–54
Cha TS, Chen CF, Yee W, Aziz A, Loh SH (2011) Cinnamic acid, coumarin and vanillin: alternative phenolic compounds for efficient Agrobacterium-mediated transformation of the unicellular green alga, Nannochloropsis sp. J Microbiol Methods 84:430–434
Chang KS, Kim J, Park H, Hong S-J, Lee C-G, Jin E (2020) Enhanced lipid productivity in AGP knockout marine microalga Tetraselmis sp. using a DNA-free CRISPR-Cas9 RNP method. Bioresour Technol 303 doi:https://doi.org/10.1016/j.biortech.2020.122932
Christian M, Cermak T, Doyle EL, Schmidt C, Zhang F, Hummel A, Bogdanove AJ, Voytas DF (2010) Targeting DNA double-strand breaks with TAL effector nucleases. Genetics 186:757–761
Coll JM (2006) Methodologies for transferring DNA into eukaryotic microalgae. Span J Agric Res:316–330
Cong L, Ran FA, Cox DM, Lin S, Barretto RPJ, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339:819–823
Couso I, Vila M, Rodriguez H, Vargas MA, León R (2011) Overexpression of an exogenous phytoene synthase gene in the unicellular alga Chlamydomonas reinhardtii leads to an increase in the content of carotenoids. Biotechnol Prog 27:54–60
Criscuolo E, Caputo V, Diotti RA, Sautto GA, Kirchenbaum GA, Clementi N (2019) Alternative methods of vaccine delivery: an overview of edible and intradermal vaccines. J Immunol Res 2019:8303648
Cui YL, Wang JF, Jiang P, Bian SG, Qin S (2010) Transformation of Platymonas (Tetraselmis) subcordiformis (Prasinophyceae, Chlorophyta) by agitation with glass beads. World J Microbiol Biotechnol 26:1653–1657
Cui Y, Qin S, Jiang P (2014) Chloroplast transformation of Platymonas (Tetraselmis) subcordiformis with the bar gene as selectable marker. PLoS One 9:e98607
Daboussi F, Leduc S, Maréchal A, Dubois G, Guyot V, Perez-Michaut C, Amato A, Falciatore A, Juillerat A, Beurdeley M, Voytas DF, Cavarec L, Duchateau P (2014) Genome engineering empowers the diatom Phaeodactylum tricornutum for biotechnology. Nat Commun 5:3831–3837
Dauvillée D, Delhaye S, Gruyer S, Slomianny C, Moretz SE, d'Hulst C, Long CA, Ball SG, Tomavo S (2010) Engineering the chloroplast targeted malarial vaccine antigens in Chlamydomonas starch granules. PLoS One 5:e0015424
Davis A, Crum LT, Corbeil LB, Hildebrand M (2017) Expression of Histophilus somni IbpA DR2 protective antigen in the diatom Thalassiosira pseudonana. Appl Microbiol Biot 101:5313–5324
Day A, Goldschmidt-Clermont M (2011) The chloroplast transformation toolbox: selectable markers and marker removal. Plant Biotechnol J 9:540–553
Dehghani J, Adibkia K, Movafeghi A, Maleki-Kakelar H, Saeedi N, Omidi Y (2020) Towards a new avenue for producing therapeutic proteins: microalgae as a tempting green biofactory. Biotechnol Adv 40:107499
Demurtas OC, Massa S, Ferrante P, Venuti A, Franconi R, Giuliano G (2013) A Chlamydomonas-derived human papillomavirus 16 E7 vaccine induces specific tumor protection. PLoS One 8:e0061473
Deng XD, Gu B, Li YJ, Hu XW, Guo JC, Fei XW (2012) The roles of acyl-CoA: diacylglycerol acyltransferase 2 genes in the biosynthesis of triacylglycerols by the green algae Chlamydomonas reinhardtii. Mol Plant 5:945–947
Dhokane D, Bhadra B, Dasgupta S (2020) CRISPR based targeted genome editing of Chlamydomonas reinhardtii using programmed Cas9-gRNA ribonucleoprotein. Mol Biol Rep. https://doi.org/10.1007/s11033-020-05922-5
Dong B, Cheng RQ, Liu QY, Wang J, Fan ZC (2018) Multimer of the antimicrobial peptide Mytichitin-a expressed in Chlamydomonas reinhardtii exerts a broader antibacterial spectrum and increased potency. J Biosci Bioeng 125:175–179
Dreesen IA, Charpin-El Hamri G, Fussenegger M (2010) Heat-stable oral alga-based vaccine protects mice from Staphylococcus aureus infection. J Biotechnol 145:273–280
Dunahay TG (1993) Transformation of Chlamydomonas reinhardtii with silicon carbide whiskers. Biotechniques 15:452-455, 457-458, 460
Dünahay TG, Jarvis EE, Zeiler KG, Roessler PG, Brown LM (1992) Genetic engineering of microalgae for fuel production. Appl Biochem Biotechnol 34:331–339
Dvir I, Chayoth R, Sod-Moriah U, Shany S, Nyska A, Stark AH, Madar Z, Arad SM (2000) Soluble polysaccharide and biomass of red microalga Porphyridium sp. alter intestinal morphology and reduce serum cholesterol in rats. Br J Nutr 84:469–476
Dvir I, Stark AH, Chayoth R, Madar Z, Arad SM (2009) Hypocholesterolemic effects of nutraceuticals produced from the red microalga Porphyridium sp in rats. Nutrients 1:156–167
Economou C, Wannathong T, Szaub J, Purton S (2014) A simple, low-cost method for chloroplast transformation of the green alga Chlamydomonas reinhardtii. Methods Mol Biol 1132:401–411
Eichler-Stahlberg A, Weisheit W, Ruecker O, Heitzer M (2009) Strategies to facilitate transgene expression in Chlamydomonas reinhardtii. Planta 229:873–883
Eilers U, Bikoulis A, Breitenbach J, Büchel C, Sandmann G (2016) Limitations in the biosynthesis of fucoxanthin as targets for genetic engineering in Phaeodactylum tricornutum. J Appl Phycol 28:123–129
Endo H, Yoshida M, Uji T, Saga N, Inoue K, Nagasawa H (2016) Stable nuclear transformation system for the coccolithophorid alga Pleurochrysis carterae. Sci Rep 6:22252
Fajardo C, Donato M, Carrasco R, Martínez-Rodríguez G, Mancera JM, Fernández-Acero FJ (2019) Advances and challenges in genetic engineering of microalgae. Rev Aquac 12:365–381
Fayyaz M, Chew KW, Show PL, Ling TC, Ng IS, Chang J-S (2020) Genetic engineering of microalgae for enhanced biorefinery capabilities. Biotechnol Adv:107554
Feng S, Feng W, Zhao L, Gu H, Li Q, Shi K, Guo S, Zhang N (2014) Preparation of transgenic Dunaliella salina for immunization against white spot syndrome virus in crayfish. Arch Virol 159:519–525
Ferenczi A, Pyott DE, Xipnitou A, Molnar A (2017) Efficient targeted DNA editing and replacement in Chlamydomonas reinhardtii using Cpf1 ribonucleoproteins and single-stranded DNA. Proc Natl Acad Sci U S A 114:13567–13572
Galarza JI, Gimpel JA, Rojas V, Arredondo-Vega BO, Henríquez V (2018) Over-accumulation of astaxanthin in Haematococcus pluvialis through chloroplast genetic engineering. Algal Res 31:291–297
Gallaher SD, Fitz-Gibbon ST, Strenkert D, Purvine SO, Pellegrini M, Merchant SS (2018) High-throughput sequencing of the chloroplast and mitochondrion of Chlamydomonas reinhardtii to generate improved de novo assemblies, analyze expression patterns and transcript speciation, and evaluate diversity among laboratory strains and wild isolates. Plant J 93:545–565
Gan Q, Jiang J, Han X, Wang S, Lu Y (2018) Engineering the chloroplast genome of oleaginous marine microalga Nannochloropsis oceanica. Front Plant Sci 9:439
Gardeva E, Toshkova R, Minkova K, Gigova L (2009) Cancer protective action of polysaccharide, derived from red microalga Porphyridium cruentum—a biological background. Biotechnol Biotechnol Equip 23:783–787
Gargouri M, Park JJ, Holguin FO, Kim MJ, Wang H, Deshpande RR, Shachar-Hill Y, Hicks LM, Gang DR (2015) Identification of regulatory network hubs that control lipid metabolism in Chlamydomonas reinhardtii. J Exp Bot 66:4551–4566
Gelvin SB (2003) Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol Mol Biol Rev 67:16–37
Geng D, Wang Y, Wang P, Li W, Sun Y (2003) Stable expression of hepatitis B surface antigen gene in Dunaliella salina (Chlorophyta). J Appl Phycol 15:451–456
Gomma AE, Lee S-K, Sun SM, Yang SH, Chung G (2015) Improvement in oil production by increasing malonyl-CoA and glycerol-3-phosphate pools in Scenedesmus quadricauda. Indian J Microbiol 55:447–455
Gong M, Bassi A (2016) Carotenoids from microalgae: a review of recent developments. Biotechnol Adv 34:1396–1412
Gourdon D, Lin Q, Oroudjev E, Hansma H, Golan Y, Arad S, Israelachvili J (2008) Adhesion and stable low friction provided by a subnanometer-thick monolayer of a natural polysaccharide. Langmuir 24:1534–1540
Gregory JA, Li F, Tomosada LM, Cox CJ, Topol AB, Vinetz JM, Mayfield S (2012) Algae-produced Pfs25 elicits antibodies that inhibit malaria transmission. PLoS One 7:e0037179
Greiner A, Kelterborn S, Evers H, Kreimer G, Sizova I, Hegemann P (2017) Targeting of photoreceptor genes in Chlamydomonas reinhardtii via zinc-finger nucleases and CRISPR/Cas9. Plant Cell 29:2498–2518
Gunasekaran B, Gothandam KM (2020) A review on edible vaccines and their prospects. Braz J Med Biol Res:53. https://doi.org/10.1590/1414-431X20198749
Gustafsson C, Govindarajan S, Minshull J (2004) Codon bias and heterologous protein expression. Trends Biotechnol 22:346–353
Hamilton ML, Haslam RP, Napier JA, Sayanova O (2014) Metabolic engineering of Phaeodactylum tricornutum for the enhanced accumulation of omega-3 long chain polyunsaturated fatty acids. Metab Eng 22:3–9
Hanschen ER, Starkenburg SR (2020) The state of algal genome quality and diversity. Algal Res 50. https://doi.org/10.1016/j.algal.2020.101968
Hawkins RL, Nakamura M (1999) Expression of human growth hormone by the eukaryotic alga, Chlorella. Curr Microbiol 38:335–341
He D-M, Qian K-X, Shen G-F, Zhang Z-F, Li Y-N, Su Z-L, Shao H-B (2007) Recombination and expression of classical swine fever virus (CSFV) structural protein E2 gene in Chlamydomonas reinhardtii chroloplasts. Colloids Surf B 55:26–30
Heitzer M, Eckert A, Fuhrmann M, Griesbeck C (2007) Influence of codon bias on the expression of foreign genes in microalgae. Adv Exp Med Biol 616:46–53
Hempel F, Lau J, Klingl A, Maier UG (2011) Algae as protein factories: expression of a human antibody and the respective antigen in the diatom Phaeodactylum tricornutum. PLoS One 6:e0028424
Hiramatsu T, Misumi O, Kuroiwa T, Nakamura S (2006) Morphological changes in mitochondrial and chloroplast nucleoids and mitochondria during the Chlamydomonas reinhardtii (Chlorophyceae) cell cycle. J Phycol 42:1048–1058
Hu Z, Zhao Z, Wu Z, Fan Z, Chen J, Wu J, Li J (2011) Successful expression of heterologous egfp gene in the mitochondria of a photosynthetic eukaryote Chlamydomonas reinhardtii. Mitochondrion 11:716–721
Hu ZL, Fan Z, Zhao ZL, Chen J, Li JC (2012) Stable expression of antibiotic-resistant gene ble from Streptoalloteichus hindustanus in the mitochondria of Chlamydomonas reinhardtii. PLoS One 7:e0035542
Ibáñez-Salazar A, Rosales-Mendoza S, Rocha-Uribe A, Ramírez-Alonso JI, Lara-Hernández I, Hernández-Torres A, Paz-Maldonado LMT, Silva-Ramírez AS, Bañuelos-Hernández B, Martínez-Salgado JL, Soria-Guerra RE (2014) Over-expression of Dof-type transcription factor increases lipid production in Chlamydomonas reinhardtii. J Biotechnol 184:27–38
Jaeger LD, Verbeek RE, Draaisma RB, Martens DE, Springer J, Eggink G, Wijffels RH (2014) Superior triacylglycerol (TAG) accumulation in starchless mutants of Scenedesmus obliquus: (I) mutant generation and characterization. Biotechnol Biofuels 7:69–69
Jarvis EE, Brown LM (1991) Transient expression of firefly luciferase in protoplasts of the green alga Chlorella ellipsoidea. Curr Genet 19:317–321
Jia X-H, Zhang C-L, Shi D-J, Zhuang M-M, Wang X, Jia R, Zhang Z-Y, Huang J, Sun Y-H, Qian W-Y, Peng G-H, He P-M (2016) Oral administration of Anabaena-expressed VP28 for both drug and food against white spot syndrome virus in shrimp. J Appl Phycol 28:1001–1009
Jiang W, Brueggeman AJ, Horken KM, Plucinak TM, Weeks DP (2014) Successful transient expression of Cas9 and single guide RNA genes in Chlamydomonas reinhardtii. Eukaryot Cell 13:1465–1469
Jiang JP, Yao CH, Cao XP, Liu YH, Xue S (2017) Characterization of starch phosphorylase from the marine green microalga (Chlorophyta) Tetraselmis subcordiformis reveals its potential role in starch biosynthesis. J Plant Phycol 218:84–93
Jin E, Polle JEW, Melis A (2001) Involvement of zeaxanthin and of the Cbr protein in the repair of photosystem II from photoinhibition in the green alga Dunaliella salina. Biochim Biophys Acta 1506:244–259
Kadono T, Kira N, Suzuki K, Iwata O, Ohama T, Okada S, Nishimura T, Akakabe M, Tsuda M, Adachi M (2015) Effect of an introduced phytoene synthase gene expression on carotenoid biosynthesis in the marine diatom Phaeodactylum tricornutum. Mar Drugs 13:5334–5357
Kajikawa M, Kinohira S, Ando A, Shimoyama M, Kato M, Fukuzawa H (2015) Accumulation of squalene in a microalga Chlamydomonas reinhardtii by genetic modification of squalene synthase and squalene epoxidase genes. PLoS One 10:e0120446
Kang NK, Jeon S, Kwon S, Koh HG, Shin S-E, Lee B, Choi G-G, Yang J-W, B-r J, Chang YK (2015) Effects of overexpression of a bHLH transcription factor on biomass and lipid production in Nannochloropsis salina. Biotechnol Biofuels 8:200–200
Kang NK, Kim EK, Kim YU, Lee B, Jeong W-J, Jeong B-R, Chang YK (2017) Increased lipid production by heterologous expression of AtWRI1 transcription factor in Nannochloropsis salina. Biotechnol Biofuels 10:1–14
Kao P-H, Ng IS (2017) CRISPRi mediated phosphoenolpyruvate carboxylase regulation to enhance the production of lipid in Chlamydomonas reinhardtii. Bioresour Technol 245:1527–1537
Karas BJ, Diner RE, Lefebvre SC, McQuaid J, Phillips AP, Noddings CM, Brunson JK, Valas RE, Deerinck TJ, Jablanovic J, Gillard JT, Beeri K, Ellisman MH, Glass JI, Hutchison CA 3rd, Smith HO, Venter JC, Allen AE, Dupont CL, Weyman PD (2015) Designer diatom episomes delivered by bacterial conjugation. Nat Commun 6:6925
Kasai Y, Harayama S (2016) Construction of marker-free transgenic strains of Chlamydomonas reinhardtii using a Cre/loxP-mediated recombinase system. PLoS One 11:e0161733
Kaye Y, Grundman O, Leu S, Zarka A, Zorin B, Didi-Cohen S, Khozin-Goldberg I, Boussiba S (2015) Metabolic engineering toward enhanced LC-PUFA biosynthesis in Nannochloropsis oceanica: overexpression of endogenous Δ12 desaturase driven by stress-inducible promoter leads to enhanced deposition of polyunsaturated fatty acids in TAG. Algal research biomass biofuels and bioproducts Algal Res 11:387–398
Khatiwada B, Kautto L, Sunna A, Sun A, Nevalainen H (2019) Nuclear transformation of the versatile microalga Euglena gracilis. Algal Res 37:178–185
Khozin-Goldberg I (2016) Lipid metabolism in microalgae. In: Borowitzka MA, Beardall J, Raven JA (eds) The physiology of microalgae. Springer, Dordrecht, pp 413–484
Kiataramgul A, Maneenin S, Purton S, Areechon N, Hirono I, Brocklehurst TW, Unajak S (2020) An oral delivery system for controlling white spot syndrome virus infection in shrimp using transgenic microalgae. Aquaculture 521
Kilian O, Benemann C, Vick B (2011) High-efficiency homologous recombination in the oil-producing alga, Nannochloropsis. Proc Natl Acad Sci U S A 108:21265–21269
Kim YG, Cha J, Chandrasegaran S (1996) Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc Natl Acad Sci U S A 93:1156–1160
Kim DH, Kim YT, Cho JJ, Bae JH, Hur SB, Hwang I, Choi TJ (2002) Stable integration and functional expression of flounder growth hormone gene in transformed microalga, Chlorella ellipsoidea. Mar Biotechnol 4:63–73
Kim J, Kwak HS, Sim SJ, Jin ES (2019) Overexpression of malic enzyme isoform 2 in Chlamydomonas reinhardtii PTS42 increases lipid production. Bioresour Technol Rep 7:100239
Kim J, Lee S, Baek K, Jin E (2020) Site-specific gene knock-out and on-site heterologous gene overexpression in Chlamydomonas reinhardtii via a CRISPR-Cas9-mediated knock-in method. Front Plant Sci 11. https://doi.org/10.3389/FPLS.2020.00306
Kindle KL (1990) High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A 87:1228–1232
Kindle KL, Schnell RA, Fernández E, Lefebvre PA (1989) Stable nuclear transformation of Chlamydomonas using the Chlamydomonas gene for nitrate reductase. J Cell Biol 109:2589–2601
Kocak DD, Josephs EA, Bhandarkar V, Adkar SS, Kwon JB, Gersbach CA (2019) Increasing the specificity of CRISPR systems with engineered RNA secondary structures. Nat Biotechnol 37:657–666
Kong F, Yamasaki T, Kurniasih SD, Hou L, Li X, Ivanova N, Okada S, Ohama T (2015) Robust expression of heterologous genes by selection marker fusion system in improved Chlamydomonas strains. J Biosci Bioeng 120:239–245
Kong F, Liang Y, Légeret B, Beyly-Adriano A, Blangy S, Haslam RP, Napier JA, Beisson F, Peltier G, Li-Beisson Y (2017) Chlamydomonas carries out fatty acid β-oxidation in ancestral peroxisomes using a bona fide acyl-CoA oxidase. Plant J 90:358–371
Kumar SV, Misquitta RW, Reddy VS, Rao BJ, Rajam MV (2004) Genetic transformation of the green alga—Chlamydomonas reinhardtii by Agrobacterium tumefaciens. Plant Sci 166:731–738
Kumar A, Falcao VR, Sayre RT (2013) Evaluating nuclear transgene expression systems in Chlamydomonas reinhardtii. Algal Res 2:321–332
Kumar A, Perrine Z, Stroff C, Postier BL, Coury DA, Sayre RT, Thomas Allnutt FC (2016) Molecular tools for bioengineering eukaryotic microalgae. Curr Biotechnol 5:93–108
Kumari S, Vira C, Lali AM, Prakash G (2020) Heterologous expression of a mutant Orange gene from Brassica oleracea increases carotenoids and induces phenotypic changes in the microalga Chlamydomonas reinhardtii. Algal Res 47:101871
Kurita T, Moroi K, Iwai M, Okazaki K, Shimizu S, Nomura S, Saito F, Maeda S, Takami A, Sakamoto A, Ohta H, Sakuma T, Yamamoto T (2020) Efficient and multiplexable genome editing using Platinum TALENs in oleaginous microalga, Nannochloropsis oceanica NIES-2145. Genes Cells 25:695–702
Kurniasih SD, Yamasaki T, Kong F, Okada S, Widyaningrum D, Ohama T (2016) UV-mediated Chlamydomonas mutants with enhanced nuclear transgene expression by disruption of DNA methylation-dependent and independent silencing systems. Plant Mol Biol 92:629–641
Kwon K-C, Verma D, Singh ND, Herzog R, Daniell H (2013) Oral delivery of human biopharmaceuticals, autoantigens and vaccine antigens bioencapsulated in plant cells. Adv Drug Deliv Rev 65:782–799
Kwon S, Kang NK, Koh HG, Shin S-E, Lee B, Jeong B-R, Chang YK (2018a) Enhancement of biomass and lipid productivity by overexpression of a bZIP transcription factor in Nannochloropsis salina. Biotechnol Bioeng 115:331–340
Kwon YM, Kim KW, Choi T-Y, Kim SY, Kim JYH (2018) Manipulation of the microalgal chloroplast by genetic engineering for biotechnological utilization as a green biofactory. World J Microbiol Biotechnol 34:183
Kwon KC, Lamb A, Fox D, Porphy Jegathese SJ (2019) An evaluation of microalgae as a recombinant protein oral delivery platform for fish using green fluorescent protein (GFP). Fish Shellfish Immunol 87:414–420
Kyriakopoulou K, Papadaki S, Krokida M (2015) Life cycle analysis of β-carotene extraction techniques. J Food Eng 167:51–58
Lao YM, Xiao L, Luo LX, Jiang JG (2014) Hypoosmotic expression of Dunaliella bardawil ζ-carotene desaturase is attributed to a hypoosmolarity-responsive element different from other key carotenogenic genes. Plant Physiol 165:359–372
León R, Couso I, Fernández E (2007) Metabolic engineering of ketocarotenoids biosynthesis in the unicelullar microalga Chlamydomonas reinhardtii. J Biotechnol 130:143–152
Li Z, Bock R (2018) Replication of bacterial plasmids in the nucleus of the red alga Porphyridium purpureum. Nat Commun 9:3451
Li YT, Han DX, Hu GR, Dauvillee D, Sommerfeld M, Ball S, Hu Q (2010a) Chlamydomonas starchless mutant defective in ADP-glucose pyrophosphorylase hyper-accumulates triacylglycerol. Metab Eng 12:387–391
Li YT, Han DX, Hu GR, Sommerfeld M, Hu Q (2010b) Inhibition of starch synthesis results in overproduction of lipids in Chlamydomonas reinhardtii. Biotechnol Bioeng 107:258–268
Li DW, Cen SY, Liu YH, Balamurugan S, Zheng XY, Alimujiang A, Yang WD, Liu JS, Li HY (2016) A type 2 diacylglycerol acyltransferase accelerates the triacylglycerol biosynthesis in heterokont oleaginous microalga Nannochloropsis oceanica. J Biotechnol 229:65–71
Li S, Ji L, Shi Q, Wu H, Fan J (2019) Advances in the production of bioactive substances from marine unicellular microalgae Porphyridium spp. Bioresour Technol 292:122048
Lichtenthaler HK, Schwender J, Disch A, Rohmer M (1997) Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate-independent pathway. FEBS Lett 400:271–274
Liu J, Gerken H, Huang J, Chen F (2013a) Engineering of an endogenous phytoene desaturase gene as a dominant selectable marker for Chlamydomonas reinhardtii transformation and enhanced biosynthesis of carotenoids. Process Biochem 48:788–795
Liu L, Wang Y, Zhang Y, Chen X, Zhang P, Ma S (2013b) Development of a new method for genetic transformation of the green alga Chlorella ellipsoidea. Mol Biotechnol 54:211–219
Liu J, Sun Z, Gerken H, Huang J, Jiang Y, Chen F (2014) Genetic engineering of the green alga Chlorella zofingiensis: a modified norflurazon-resistant phytoene desaturase gene as a dominant selectable marker. Appl Microbiol Biotechnol 98:5069–5079
Liu J, Sun Z, Mao X, Gerken H, Wang X, Yang W (2019) Multiomics analysis reveals a distinct mechanism of oleaginousness in the emerging model alga Chromochloris zofingiensis. Plant J 98:1060–1077
Lumbreras V, Stevens DR, Purton S (1998) Efficient foreign gene expression in Chlamydomonas reinhardtii mediated by an endogenous intron. Plant J 14:441–447
Ma YH, Wang X, Niu Y-F, Yang ZK, Zhang MH, Wang ZM, Yang WD, Liu JS, Li HY (2014) Antisense knockdown of pyruvate dehydrogenase kinase promotes the neutral lipid accumulation in the diatom Phaeodactylum tricornutum. Microb Cell Factories 13:100–100
Manandhar-Shrestha K, Hildebrand M (2015) Characterization and manipulation of a DGAT2 from the diatom Thalassiosira pseudonana: improved TAG accumulation without detriment to growth, and implications for chloroplast TAG accumulation. Algal Res 12:239–248
Marquez-Escobar VA, Banuelos-Hernandez B, Rosales-Mendoza S (2018) Expression of a Zika virus antigen in microalgae: towards mucosal vaccine development. J Biotechnol 282:86–91
Mayfield SP, Franklin SE (2005) Expression of human antibodies in eukaryotic micro-algae. Vaccine 23:1828–1832
Mayfield SP, Franklin SE, Lerner RA (2003) Expression and assembly of a fully active antibody in algae. Proc Natl Acad Sci U S A 100:438–442
McClure DD, Luiz A, Gerber B, Barton GW, Kavanagh JM (2018) An investigation into the effect of culture conditions on fucoxanthin production using the marine microalgae Phaeodactylum tricornutum. Algal Res 29:41–48
Mendes-Pinto MM, Raposo MFJ, Bowen J, Young AJ, Morais R (2001) Evaluation of different cell disruption processes on encysted cells of Haematococcus pluvialis: effects on astaxanthin recovery and implications for bio-availability. J Appl Phycol 13:19–24
Minoda A, Sakagami R, Yagisawa F, Kuroiwa T, Tanaka K (2004) Improvement of culture conditions and evidence for nuclear transformation by homologous recombination in a red alga, Cyanidioschyzon merolae 10D. Plant Cell Physiol 45:667–671
Mizutani O, Masaki K, Gomi K, Iefuji H (2012) Modified Cre-loxP recombination in Aspergillus oryzae by direct introduction of Cre recombinase for marker gene rescue. Appl Environ Microbiol 78:4126–4133
Mohammed A, Ali M, Kawasaki T, Yamada T (2007) Characterization of a chitinase gene encoded by virus-sensitive Chlorella strains and expressed during virus infection. Arab J Biotech 10:81–96
Molina-Márquez A, Vila M, Rengel R, Fernández E, García-Maroto F, Vigara J, León R (2020) Validation of a new multicistronic plasmid for the efficient and stable expression of transgenes in microalgae. Int J Mol Sci 21:718–732
Morikawa T, Uraguchi Y, Sanda S, Nakagawa S, Sawayama S (2018) Overexpression of DnaJ-like chaperone enhances carotenoid synthesis in Chlamydomonas reinhardtii. Appl Biochem Biotechnol 184:80–91
Muñoz CF, Sturme MHJ, D'Adamo S, Weusthuis RA, Wijffels RH (2019) Stable transformation of the green algae Acutodesmus obliquus and Neochloris oleoabundans based on E coli conjugation. Algal Res:39. https://doi.org/10.1016/j.algal.2019.101453
Naduthodi MIS, Mohanraju P, Südfeld C, D’Adamo S, Barbosa MJ, Jvd O (2019) CRISPR-Cas ribonucleoprotein mediated homology-directed repair for efficient targeted genome editing in microalgae Nannochloropsis oceanica IMET1. Biotechnol Biofuels 12:1–11
Ng IS, Tan SI, Kao PH, Chang YK, Chang JS (2017) Recent developments on genetic engineering of microalgae for biofuels and bio-based chemicals. Biotechnol J 12:e1600644
Ng IS, Keskin BB, Tan SI (2020) A critical review of genome editing and synthetic biology applications in metabolic engineering of microalgae and cyanobacteria. Biotechnol J 12:e1600644
Niu YF, Zhang MH, Xie WH, Li JN, Gao YF, Yang WD, Liu JS, Li HY (2011) A new inducible expression system in a transformed green alga, Chlorella vulgaris. Genet Mol Res 10:3427–3434
Niu YF, Yang ZK, Zhang MH, Zhu CC, Yang WD, Liu JS, Li HY (2012) Transformation of diatom Phaeodactylum tricornutum by electroporation and establishment of inducible selection marker. Biotechniques 52:1–3
Nymark M, Sharma AK, Sparstad T, Bones AM, Winge P (2016) A CRISPR/Cas9 system adapted for gene editing in marine algae. Sci Rep 6:24951–24951
Ohta S, Nakagawara S, Hirai S, Miyagishima K, Horiguchi G, Kodama H (2018) The 5′ UTR intron-mediated enhancement of constitutive splicing of the tobacco microsome ω-3 fatty acid desaturase gene. Plant Biotechnol Rep 12:105–114
Park S, Nguyen THT, Jin E (2019) Improving lipid production by strain development in microalgae: strategies, challenges and perspectives. Bioresour Technol 292:121953
Peng KT, Zheng CN, Xue J, Chen XY, Yang WD, Liu JS, Wb B, Li HY (2014) Delta 5 fatty acid desaturase upregulates the synthesis of polyunsaturated fatty acids in the marine diatom Phaeodactylum tricornutum. J Agric Food Chem 62:8773–8776
Periz G, Keller LR (1997) DNA elements regulating alpha1-tubulin gene induction during regeneration of eukaryotic flagella. Mol Cell Biol 17:3858
Perozeni F, Cazzaniga S, Baier T, Zanoni F, Zoccatelli G, Lauersen KJ, Wobbe L, Ballottari M (2020) Turning a green alga red: engineering astaxanthin biosynthesis by intragenic pseudogene revival in Chlamydomonas reinhardtii. Plant Biotechnol J:1–15
Picariello T, Hou Y, Kubo T, McNeill NA, Yanagisawa HA, Oda T, Witman GB (2020) TIM, a targeted insertional mutagenesis method utilizing CRISPR/Cas9 in Chlamydomonas reinhardtii. PLoS One 15:e0232594
Poliner E, Pulman JA, Zienkiewicz K, Childs K, Benning C, Farré EM (2018) A toolkit for Nannochloropsis oceanica CCMP1779 enables gene stacking and genetic engineering of the eicosapentaenoic acid pathway for enhanced long-chain polyunsaturated fatty acid production. Plant Biotechnol J 16:298–309
Porse H, Bixler R (2017) The seaweed hydrocolloid industry: 2016 updates, requirements, and outlook. J Appl Phycol 29:2187–2200
Pralhad Rathod J, M. Gade R, Rathod DR, Dudhare M (2017) A review on molecular tools of microalgal genetic transformation and their application for overexpression of different genes. Int J Curr Microbiol Appl Sci 6:3191–3207
Prommuak C, Pavasant P, Quitain AT, Goto M, Shotipruk A (2013) Simultaneous production of biodiesel and free lutein from Chlorella vulgaris. Chem Eng Technol 36:733–739
Rakkhumkaew N, Kawasaki T, Fujie M, Yamada T (2013) Prolonged synthesis of hyaluronan by Chlorella cells infected with chloroviruses. J Biosci Bioeng 115:527–531
Ramazanov A, Ramazanov Z (2006) Isolation and characterization of a starchless mutant of Chlorella pyrenoidosa STL-PI with a high growth rate, and high protein and polyunsaturated fatty acid content. Phycol Res 54:255–259
Rasala BA, Mayfield SP (2015) Photosynthetic biomanufacturing in green algae; production of recombinant proteins for industrial, nutritional, and medical uses. Photosynth Res 123:227–239
Rasala BA, Muto M, Lee PA, Jager M, Cardoso RMF, Behnke CA, Kirk P, Hokanson CA, Crea R, Mendez M, Mayfield SP (2010) Production of therapeutic proteins in algae, analysis of expression of seven human proteins in the chloroplast of Chlamydomonas reinhardtii. Plant Biotechnol J 8:719–733
Rasala BA, Lee PA, Shen Z, Briggs SP, Mendez M, Mayfield SP (2012) Robust expression and secretion of Xylanase1 in Chlamydomonas reinhardtii by fusion to a selection gene and processing with the FMDV 2A peptide. PLoS One 7:e0043349
Reiter Y, Wright AF, Tonge DW, Pastan I (1996) Recombinant single-chain and disulfide-stabilized Fv-immunotoxins that cause complete regression of a human colon cancer xenograft in nude mice. Int J Cancer 67:113–123
Remacle C, Cardol P, Coosemans N, Gaisne M, Bonnefoy N (2006) High-efficiency biolistic transformation of Chlamydomonas mitochondria can be used to insert mutations in complex I genes. Proc Natl Acad Sci U S A 103:4771
Rengel R, Smith RT, Haslam RP, Sayanova O, Vila M, León R (2018) Overexpression of acetyl-CoA synthetase (ACS) enhances the biosynthesis of neutral lipids and starch in the green microalga Chlamydomonas reinhardtii. Algal Res 31:183–193
Roessler PG, Bleibaum JL, Thompson GA, Ohlrogge JB (1994) Characteristics of the gene that encodes acetyl-CoA carboxylase in the diatom Cyclotella cryptica. Ann N Y Acad Sci 721:250–256
Rossi F, De Philippis R (2016) Exocellular polysaccharides in microalgae and cyanobacteria: Chemical features, role and enzymes and genes involved in their biosynthesis. In: Borowitzka MA, Beardall J, Raven JA (eds) The physiology of microalgae. Springer, Dordrecht, pp 565–590
Sakaguchi T, Nakajima K, Matsuda Y (2011) Identification of the UMP synthase gene by establishment of uracil auxotrophic mutants and the phenotypic complementation system in the marine diatom Phaeodactylum tricornutum. Plant Physiol 156:78–89
Sakamoto H, Torada H, Goto K, Nakamura Y, Nakano T, Yamaguchi T, Sato M, Saito T, Taniguchi A, Yokoyama T, Kan-No N, Nagahisa E (2003) Biological activity of the polysaccharide produced by the marine phytoplankton Porphyridium sp. and additive effect of slag on the polysaccharide production. J Iron Steel Inst Jpn 89:475–481
Sanitha M, Radha S, Fatima AA, Devi SG, Ramya M (2014) Agrobacterium-mediated transformation of three freshwater microalgal strains. Pol J Microbiol 63:387–392
Schroda M, Vallon O, Wollman F-A, Beck CF (1999) A chloroplast-targeted heat shock protein 70 (HSP70) contributes to the photoprotection and repair of photosystem II during and after photoinhibition. Plant Cell 11:1165–1178
Schroda M, Blöcker D, Beck CF (2000) The HSP70A promoter as a tool for the improved expression of transgenes in Chlamydomonas. Plant J 21:121–131
Schroda M, Beck CF, Vallon O (2002) Sequence elements within an HSP70 promoter counteract transcriptional transgene silencing in Chlamydomonas. Plant J 31:445–455
Scranton MA, Ostrand JT, Georgianna DR, Lofgren SM, Li D, Ellis RC, Carruthers DN, Dräger A, Masica DL, Mayfield SP (2016) Synthetic promoters capable of driving robust nuclear gene expression in the green alga Chlamydomonas reinhardtii. Algal Res 15:135–142
Serif M, Lepetit B, Weißert K, Kroth PG, Rio Bartulos C (2017) A fast and reliable strategy to generate TALEN-mediated gene knockouts in the diatom Phaeodactylum tricornutum. Algal Res 23:186–195
Serif M, Dubois G, Finoux A-L, Teste M-A, Jallet D, Daboussi F (2018) One-step generation of multiple gene knock-outs in the diatom Phaeodactylum tricornutum by DNA-free genome editing. Nat Commun 9:3924
Shin SE, Lim JM, Koh HG, Kim EK, Kang NK, Jeon S, Kwon S, Shin WS, Lee B, Hwangbo K, Kim J, Ye SH, Yun JY, Seo H, Oh HM, Kim KJ, Kim JS, Jeong WJ, Chang YK, Jeong BR (2016) CRISPR/Cas9-induced knockout and knock-in mutations in Chlamydomonas reinhardtii. Sci Rep 6:27810
Shin YS, Jeong J, Nguyen THT, Kim JYH, Jin E, Sim SJ (2019) Targeted knockout of phospholipase A2 to increase lipid productivity in Chlamydomonas reinhardtii for biodiesel production. Bioresour Technol 271:368–374
Siaut M, Cuiné S, Cagnon C, Fessler B, Nguyen M, Carrier P, Beyly A, Beisson F, Triantaphylidès C, Li-Beisson Y, Peltier G (2011) Oil accumulation in the model green alga Chlamydomonas reinhardtii : characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnol 11:7–7
Simon DP, Anila N, Gayathri K, Sarada R (2016) Heterologous expression of β-carotene hydroxylase in Dunaliella salina by Agrobacterium -mediated genetic transformation. Algal Res 18:257–265
Sizova I, Greiner A, Awasthi M, Kateriya S, Hegemann P (2013) Nuclear gene targeting in Chlamydomonas using engineered zinc-finger nucleases. Plant J 73:873–882
Sodeinde OA, Kindle KL (1993) Homologous recombination in the nuclear genome of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A 90:9199–9203
Specht EA, Mayfield SP (2014) Algae-based oral recombinant vaccines. Front Microbiol 5:60
Srinivasan R, Babu S, Gothandam KM (2017) Accumulation of phytoene, a colorless carotenoid by inhibition of phytoene desaturase (PDS) gene in Dunaliella salina V-101. Bioresour Technol 242:311–318
Steinbrenner J, Sandmann G (2006) Transformation of the green alga Haematococcus pluvialis with a phytoene desaturase for accelerated astaxanthin biosynthesis. Appl Environ Microbiol 72:7477–7484
Sun M, Qian K, Su N, Chang H, Liu J, Shen G (2003) Foot-and-mouth disease virus VP1 protein fused with cholera toxin B subunit expressed in Chlamydomonas reinhardtii chloroplast. Biotechnol Lett 25:1087–1092
Sun H, Zhao W, Mao X, Li Y, Wu T, Chen F (2018a) High-value biomass from microalgae production platforms: strategies and progress based on carbon metabolism and energy conversion. Biotechnol Biofuels 11:254–254
Sun T, Li S, Song X, Diao J, Chen L, Zhang W (2018b) Toolboxes for cyanobacteria: recent advances and future direction. Biotech Adv 36:1293–1307
Sun XM, Ren LJ, Zhao QY, Ji XJ, Huang H (2019) Enhancement of lipid accumulation in microalgae by metabolic engineering. Biochim Biophys Acta Mol Cell Biol Lipids 1864:552–566
Surzycki R, Greenham K, Kitayama K, Dibal F, Wagner R, Rochaix J-D, Ajam T, Surzycki S (2009) Factors effecting expression of vaccines in microalgae. Biologicals 37:133–138
Takahashi K, Ide Y, Hayakawa J, Yoshimitsu Y, Fukuhara I, Abe J, Kasai Y, Harayama S (2018) Lipid productivity in TALEN-induced starchless mutants of the unicellular green alga Coccomyxa sp. strain Obi. Algal Res 32:300–307
Talyshinsky MM, Souprun YY, Huleihel MM (2002) Anti-viral activity of red microalgal polysaccharides against retroviruses. Cancer Cell Int 2:8–8
Te MR, Lohuis, Miller DJ (1998) Genetic transformation of dinoflagellates (Amphidinium and Symbiodinium): expression of GUS in microalgae using heterologous promoter constructs. Plant J 13:427–435
Teng C, Qin S, Liu J, Yu D, Liang C, Tseng C (2002) Transient expression of lacZ in bombarded unicellular green alga Haematococcus pluvialis. J Appl Phycol 14:497–500
Tran M, Henry RE, Siefker D, Van C, Newkirk G, Kim J, Bui J, Mayfield SP (2013) Production of anti-cancer immunotoxins in algae: ribosome inactivating proteins as fusion partners. Biotechnol Bioeng 110:2826–2835
Tran Q-G, Cho K, Kim U, Yun J-H, D-h C, Heo J, Park S-B, Kim JW, Lee YJ, Ramanan R, Kim H-S (2019) Enhancement of β-carotene production by regulating the autophagy-carotenoid biosynthesis seesaw in Chlamydomonas reinhardtii. Bioresour Technol 292:121937
Trentacoste EM, Shrestha RP, Smith SR, Glé C, Hartmann AC, Hildebrand M, Gerwick WH (2013) Metabolic engineering of lipid catabolism increases microalgal lipid accumulation without compromising growth. Proc Natl Acad Sci U S A 110:19748–19753
Ungerer J, Pakrasi HB (2016) Cpf1 is a versatile tool for CRISPR genome editing across diverse species of cyanobacteria. Sci Rep 6:39681–39681
Vanier G, Stelter S, Vanier J, Hempel F, Maier UG, Lerouge P, Ma J, Bardor M (2018) Alga-made anti-hepatitis B antibody binds to human Fcγ receptors. Biotechnol J 13:1700496
Walsh G (2018) Biopharmaceutical benchmarks 2018. Nat Biotechnol 36:1136–1145
Wang TY, Xue LX, Ji X, Wang YF (2007) Matrix attachment region increased reporter gene expression in stably transformed Dunaliella salina. Paper presented at the 2006 ASAE Annual Meeting, 2007-08-20
Wang QT, Lu YD, Xin Y, Wei L, Huang S, Xu J (2016) Genome editing of model oleaginous microalgae Nannochloropsis spp. by CRISPR/Cas9. Plant J 88:1071–1081
Wang X, Dong H-P, Wei W, Balamurugan S, Yang W-D, Liu J-S, Li H-Y (2018a) Dual expression of plastidial GPAT1 and LPAT1 regulates triacylglycerol production and the fatty acid profile in Phaeodactylum tricornutum. Biotechnol Biofuels 11:1–14
Wang X, Wei W, Li N-J, Yuan W, Ding Y, Yang W-D, Liu J-S, Balamurugan S, Li H-Y (2018b) Heterogeneous expression of human PNPLA3 triggers algal lipid accumulation and lipid droplet enlargement. Algal Res 31:276–281
Wang X, Wei H, Mao X, Liu J (2019) Proteomics analysis of lipid droplets from the oleaginous alga Chromochloris zofingiensis reveals novel proteins for lipid metabolism. Genom Proteom Bioinformat 17:260–272
Wannathong T, Waterhouse JC, Young RE, Economou CK, Purton S (2016) New tools for chloroplast genetic engineering allow the synthesis of human growth hormone in the green alga Chlamydomonas reinhardtii. Appl Microbiol Biotechnol 100:5467–5477
Wei H, Shi Y, Ma X, Pan Y, Hu H, Li Y, Luo M, Gerken H, Liu J (2017a) A type-I diacylglycerol acyltransferase modulates triacylglycerol biosynthesis and fatty acid composition in the oleaginous microalga, Nannochloropsis oceanica. Biotechnol Biofuels 10:174
Wei L, Wang Q, Xin Y, Lu Y, Xu J (2017b) Enhancing photosynthetic biomass productivity of industrial oleaginous microalgae by overexpression of RuBisCO activase. Algal Res 27:366–375
Weyman PD, Beeri K, Lefebvre SC, Rivera J, McCarthy JK, Heuberger AL, Peers G, Allen AE, Dupont CL (2015) Inactivation of Phaeodactylum tricornutum urease gene using transcription activator-like effector nuclease-based targeted mutagenesis. Plant Biotechnol J 13:460–470
Wu J, Hu Z, Wang C, Li S, Lei A (2008) Efficient expression of green fluorescent protein (GFP) mediated by a chimeric promoter in Chlamydomonas reinhardtii. Chin J Oceanol Limnol 26:242–247
Xin Y, Lu Y, Lee YY, Wei L, Jia J, Wang Q, Wang D, Bai F, Hu H, Hu Q, Liu J, Li Y, Xu J (2017) Producing designer oils in industrial microalgae by rational modulation of co-evolving type-2 diacylglycerol acyltransferases. Mol Plant 10:1523–1539
Xue J, Niu Y-F, Huang T, Yang W-D, Liu J-S, Li H-Y (2015) Genetic improvement of the microalga Phaeodactylum tricornutum for boosting neutral lipid accumulation. Metab Eng 27:1–9
Xue J, Balamurugan S, Li D-W, Liu Y-H, Zeng H, Wang L, Yang W-D, Liu J-S, Li H-Y (2017) Glucose-6-phosphate dehydrogenase as a target for highly efficient fatty acid biosynthesis in microalgae by enhancing NADPH supply. Metab Eng 41:212–221
Yan J, Cheng R, Lin X, You S, Li K, Rong H, Ma Y (2013) Overexpression of acetyl-CoA synthetase increased the biomass and fatty acid proportion in microalga Schizochytrium. Appl Microbiol Biotechnol 97:1933–1939
Yan J, Kuang Y, Gui X, Han X, Yan Y (2019) Engineering a malic enzyme to enhance lipid accumulation in Chlorella protothecoides and direct production of biodiesel from the microalgal biomass. Biomass Bioenergy 122:298–304
Yao Y, Lu Y, Peng K-T, Huang T, Niu Y-F, Xie W-H, Yang W-D, Liu J-S, Li H-Y (2014) Glycerol and neutral lipid production in the oleaginous marine diatom Phaeodactylum tricornutum promoted by overexpression of glycerol-3-phosphate dehydrogenase. Biotechnol Biofuels 7:110–119
Yohn C, Mendez M, Behnke C, Brand A (2011) Stress-induced lipid trigger. European Patent EP2531600A1
Yoshimitsu Y, Abe J, Harayama S (2018) Cas9-guide RNA ribonucleoprotein-induced genome editing in the industrial green alga Coccomyxa sp. strain KJ. Biotechnol Biofuels 11:326
Zäuner S, Jochum W, Bigorowski T, Benning C (2012) A cytochrome b5-containing plastid-located fatty acid desaturase from Chlamydomonas reinhardtii. Eukaryot Cell 11:856–863
Zhang J, Sun Z, Sun P, Chen T, Chen F (2014a) Microalgal carotenoids: beneficial effects and potential in human health. Food Funct 5:413–425
Zhang R, Patena W, Armbruster U, Gang SS, Blum SR, Jonikas MC (2014b) High-throughput genotyping of green algal mutants reveals random distribution of mutagenic insertion sites and endonucleolytic cleavage of transforming DNA. Plant Cell 26:1398–1409
Zhong QW, Wei B, Wang SJ, Ke SZ, Chen JW, Zhang HW, Wang H (2019) The antioxidant activity of polysaccharides derived from marine organisms: an overview. Mar Drugs 17:674
Zhou X, Zhang X, Boualavong J, Durney AR, Wang T, Kirschner S, Wentz M, Mukaibo H (2017) Electrokinetically controlled fluid injection into unicellular microalgae. Electrophoresis 38:2587–2591
Funding
This work was sponsored by National Key Research and Development Project of China 2019YFA0906300 and 2020YFA0907304, Natural Science Foundation of Shandong Province ZR2019ZD17, National Natural Science Foundation of China 31872608, Natural Science Foundation of Shanghai 18ZR1410100, Funding Project of the State Key Laboratory of Bioreactor Engineering, and Open Funding Project of the State Key Laboratory of Marine Resource Utilization in South China Sea (Hainan University) 2019002.
Author information
Authors and Affiliations
Contributions
Jianhua Fan: conceptualization, project administration, supervision. Pengcheng Fu: project administration, supervision. Qianwen Shi: investigation, writing—original draft, writing—review and editing. Cheng Chen: investigation, writing—original draft, writing—review and editing. Wei Zhang: writing—original draft. Ping Wu: writing—original draft. Hui Wu: supervision. Haizhen Wu: supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Shi, Q., Chen, C., Zhang, W. et al. Transgenic eukaryotic microalgae as green factories: providing new ideas for the production of biologically active substances. J Appl Phycol 33, 705–728 (2021). https://doi.org/10.1007/s10811-020-02350-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02350-7