Abstract
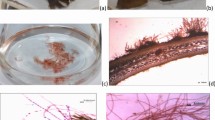
The selection of unialgal culture strains suitable as sources of algal hemagglutinins is one of the important factors for success in algal component production. To obtain suitable unialgal culture strains, several Gracilariopsis chorda and Gracilaria vermiculophylla strains were surveyed for hemagglutinating activity, relative growth rates, and fertility difficulty. Fertile tetrasporophyte samples of naturally occurring G. chorda and G. vermiculophylla were collected at each of three different collection sites around Shikoku Island in southwest Japan. Unialgal culture strains were started from isolated tetraspores obtained from each naturally occurring plant. The hemagglutinating activities in algal extracts of the three G. chorda strains (3100–7000 units mg−1) were higher than those of the three G. vermiculophylla strains (920–980 units mg−1). The daily growth rates of the G. chorda strains (8.0–13.8% day−1 at 22 °C) were higher than those of the G. vermiculophylla strains (4.1–4.4% day−1 at 22 °C). Among the unialgal culture strains tested, the one started from isolated tetraspores from G. chorda growing in the Katsuura River had the highest hemagglutinating activity and relative growth rate. This strain also did not become fertile even after a period of 3 years of culture with aeration at 22 °C, in a 14-h light–10-h dark cycle at 60 μmol photons m−2 s−1. Thus, the unialgal culture strain started from isolated tetraspores from G. chorda growing in the Katsuura River seems to be a useful source for hemagglutinin production.



Similar content being viewed by others
References
Beer S, Levy I (1983) Effects of photon fluence rate and light spectrum composition on growth, photosynthesis and pigment relations in Gracilaria sp. J Phycol 19:516–522
Bird NL, Chen LCM, McLachlan J (1979) Effects of temperature, light and salinity on growth in culture of Chondrus crispus, Furcellaria lumbricalis, Gracilaria tikvahiae (Gigartinales, Rhodophyta) and Fucus serratus (Fucales, Phaeophyta). Bot Mar 22:521–527
Chirapart A, Ohno M, Sawamura M, Kusunose H (1994) Effect of temperature on growth rate and agar quality of a new member of Japanese Gracilaria in Tosa Bay, sourthern Japan. Jpn J Phycol (Sorui) 42:325–329
Collén J, Guisle-Marsollier I, Léger JJ, Boyen C (2007) Response of the transcriptome of the intertidal red seaweed Chondrus crispus to controlled and natural stresses. New Phytol 176:45–55
Dickson DM, Jones RGW, Davenport J (1980) Steady state osmotic adaptation in Ulva lactuca. Planta 150:158–165
Dittami SM, Gravot A, Goulitquer S, Rousvoal S, Peters AF, Bouchereau A, Boyen C, Tonon T (2012) Towards deciphering dynamic changes and evolutionary mechanisms involved in the adaptation to low salinities in Ectocarpus (brown algae). Plant J 71:366–377
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Falkenberg M, Nakano E, Zambotti-Villela L, Zatelli GA, Philippus AC, Imamura KB, Velasquez AMA, Freitas RP, de Freitas TL, Colepicolo P, Graminha MAS (2019) Bioactive compounds against neglected diseases isolated from macroalgae: a review. J Appl Phycol 31:797–823
Hori K, Miyazawa K, Ito K (1981) Hemagglutinins in marine algae. Bull J Soc Sci Fish 47:793–798
Ichihara K, Arai S, Shimada S (2009) cDNA cloning of a lectin-like gene preferentially expressed in freshwater from the macroalga Ulva limnetica (Ulvales, Chlorophyta). Phycol Res 57:104–110
Kain JM (1987) Seasonal growth and photoinhibition in Plocamium cartilagineum (Rhodophyta) off the Isle of Man. Phycologia 26:88–99
Kakita H, Kitamura T (2003) Hemagglutinating activity in the cultivated red alga Gracilaria chorda. In: Chapman ARO, Anderson RJ, Vreeland VJ, Davision IR (eds) Proceedings of the 17th international seaweed symposium. Oxford University Press, New York, pp 175–182
Kakita H, Fukuoka S, Obika H, Li ZF, Kamishima H (1997) Purification and properties of a high molecular weight hemagglutinin from the red alga, Gracilaria verrucosa. Bot Mar 40:241–247
Kakita H, Fukuoka H, Obika H, Kamishima H (1999) Isolation and characterization of a fourth hemagglutinin from the red alga, Gracilaria verrucosa. J Appl Phycol 11:49–56
Kakita H, Kamishima H, Chirapart A, Ohno M (2003) Marine biopolymers from the red algae, Gracilaria spp. In: Fingerrman M, Nagabhushanam R (eds) Recent advances in marine biotechnology. Science Publishers, Enfield, pp 79–109
Kanoh H, Kitamura T, Kobayashi Y (1992) A sulfated proteoglycan from the red alga Gracilaria verrucosa is a hemagglutinin. Comp Biochem Physiol 102B:445–449
Kocourek J (1986) Historical background. In: Sharon N, Goldstain IJ (eds) IE Liener. The lectins. Academic Press, New York, pp 1–32
Laing WA, Christeller JT, Terzaghi BE (1989) The effect of temperature, photon flux density and nitrogen on growth of Gracilaria sordida Nelson (Rhodophyta). Bot Mar 32:439–445
Lapointe BE (1981) The effects of light and nitrogen on growth, pigment content, and biochemical composition of Gracilaria foliifera v. angustissima (Gigartinales, Rhodophyta). J Phycol 17:90–95
Lowry OH, Rosebrough NJ, Farr AL, Randall RL (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
McLachlan J, Bird CJ (1984) Geographical and experimental assessment of the distribution of Gracilaria species (Rhodophyta, Gigartinales) in relation to temperature. Helgol Meeresun 38:319–334
Mostaert AS, Karsten UF, King RJ (1995) Physiological responses of Caloglossa leprieurii (Ceramiales, Rhodophyta) to salinity stress. Phycol Res 43:215–222
Nelson WA (1989) Phenology of Gracilaria sordida W. Nelson populations. Reproductive status, plant and population size. Bot Mar 32:41–51
Ogata E, Matsui T, Nakamura H (1972) The life cycle of Gracilaria verrucosa (Rhodophyceae, Gigartinales) in vitro. Phycologia 11:75–80
Provasoli L (1968) Media and prospects for the cultivation of marine algae. In: Watanabe A, Hattori A (eds) Cultures and collections of algae. Jap. Soc. Plant Physiol, Tokyo, pp 63–75
Shiomi K, Yamanala H, Kikuchi T (1981) Purification and physicochemical properties of a hemagglutinin (GVA-1) in the red alga Gracilaria verrucosa. Bull J Soc Sci Fish 47:1079–1084
Smit AJ (2004) Medicinal and pharmaceutical uses of seaweed natural products: a review. J Appl Phycol 16:245–262
Takahashi Y, Katagiri S (1987) Seasonal variation of the hemagglutinating activities in the red alga Gracilaria verrucosa. Nippon Suisan Gakkaishi 53:2133–2137
Teo SS, Ho CL, Teoh S, Rahim RA, Phang SM (2009) Transcriptomic analysis of Gracilaria Changii (Rhodophyta) in response to hyper- and hypoosmotic stresses. J Phycol 45:1093–1099
Weig A, Deswarte C, Chrispeels M (1997) The major intrinsic protein family of Arabidopsis has 23 members that from three distinct groups with functional aquaporins in each group. Plant Physiol 114:1347–1357
Winer BJ, Brown DR, Michels KM (1991) Statistical principles in experimental design, 3rd edn. McGraw-Hill, New York, pp 153–198
Yamamoto H, Sasaki J (1987) Crossing experiments between populations and so-called Gracilaria verrucosa (Huds.) Papenfuss from two localities, Shinori and Kikonai in Hokkaido. Bull Fac Hokkaido Univ 38:335–338
Yokoya NS, Oliveira EC (1992) Temperature responses of economically important red algae and their potential for mariculture in Brazilian waters. J Appl Phycil 4:339–345
Yokoya NS, Kakita H, Obika H, Kitamura T (1999) Effects of environmental factors and plant growth regulators on growth of the red alga Gracilaria vermiculophylla from Shikoku Island, Japan. Hydrobiologia 398:339–347
Yoshida T, Yoshinaga K (2010) Check list of marine algae of Japan (revised in 2010). Jpn J Phycol (Sorui) 58:69–122
Yoshida T, Yoshinaga K, Nakajima Y (2000) Check list of marine algae of Japan (revised in 2000). Jpn J Phycol (Sorui) 48:113–166
Acknowledgments
We are grateful to Emeritus professor Dr. Hirotoshi Yamamoto of Hokkaido University and Professor Dr. Ryuta Terada of Kagoshima University for identifying G. chorda and G. vermiculophylla and for giving advice about unialgal culture procedure.
Funding
This research was partially supported by the Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research (C), 16K00596, 2016.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kakita, H., Yanaoka, N. & Obika, H. Suitable unialgal strains of Gracilariopsis chorda and Gracilaria vermiculophylla for hemagglutinin production. J Appl Phycol 32, 2397–2406 (2020). https://doi.org/10.1007/s10811-019-02024-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-02024-z