Abstract
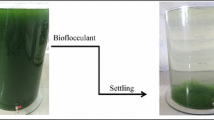
Microalgal biomass production has been investigated since the 1950s by academic and industry sectors due to its potential and for biodiesel production. One of the main bottlenecks in microalgae production is biomass recovery and its separation from aqueous medium. For this reason, the use of a flocculation process is an important step to promote microalgae recovery in large scale. Here we provide a strategy for the harvesting of microalgal biomass using chitosan as flocculating agent in pilot scale cultures performed in flat plate photobioreactor. The results show that chitosan was effective in inducing microalgae flocs and separating them by settling. In the jar test scale the best clarification efficiency was around 99% and when scaled up using a 100-L photobioreactor, the biomass recovery efficiency was close to 98%. Comparison of the compositions of biomasses obtained by flocculation and centrifugation indicates no significant differences in terms of carbohydrates, proteins, lipids, and ashes, showing that the flocculation process is not affecting the biomass characteristics and its potential biotechnological applications. Based on the presented results, flocculation using chitosan as flocculant agent can be considered as an efficient method to harvest Desmodesmus subspicatus biomass cultured in pilot scale photobioreactors.







Similar content being viewed by others
References
Ahmad AL, Yasin NHM, Derek CJC, Lim JK (2011) Optimization of microalgae coagulation process using chitosan. Chem Eng J 173:879–882
APHA (2005) Standard methods for the examination of water and waste water, 21st edn. American Public Health Association, Washington
Atkins PW (1990) Physical chemistry. WH Freeman and Company, New York
Bach Q-V, Chen W-H (2017) Pyrolysis characteristics and kinetics of microalgae via thermogravimetric analysis (TGA): a state-of-the-art review. Bioresour Technol 246:88–100
Bligh EG, Dyer WJ (1959) A rapid method for total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Brennan L, Owende P (2010) Biofuels from microalgae - a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sust Energ Rev 14:557–577
Chatsungnoen T, Chisti Y (2016) Oil production by six microalgae: impact of flocculants and drying on oil recovery from the biomass. J Appl Phycol 28:2697–2705
Chen L, Wang C, Wang W, Wei J (2013) Optimal conditions of different flocculation methods for harvesting Scenedesmus sp. cultivated in an open-pound system. Bioresour Technol 133:9–15
Correa DO, Santos B, Dias FG, Vargas JVC, Mariano AB, Balmant W, Rosa MP, Savi DC, Kava V, Glienke C, Ordonez JC (2017) Enhanced biohydrogen production from microalgae by diesel engine hazardous emissions fixation. Int J Hydrog Energy 42:21463–21475
Divakaran R, Pillai VNS (2002) Flocculation of algae using chitosan. J Appl Phycol 14:419–422
Dong C, Chen W, Liu C (2014) Flocculation of algal cells by amphoteric chitosan based flocculant. Bioresour Technol 170:239–247
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Escapa C, Coimbra RN, Paniagua S, García AI, Otero M (2017) Comparison of the culture and harvesting of Chlorella vulgaris and Tetradesmus obliquus for the removal of pharmaceuticals from water. J Appl Phycol 29:1179–1193
Gerchman Y, Vasker B, Tavasi M, Mishael Y, Kinel-Tahan Y, Yehoshuan Y (2017) Effective harvesting of microalgae: comparison of different polymeric flocculants. Bioresour Technol 228:141–146
Gerde JA, Yao L, Lio JY, Wen Z, Wang T (2014) Microalgae flocculation: impact of flocculant type, algae species and cell concentration. Algal Res 3:30–35
Grima EM, Belarbi EH, Fernández FGA, Medina AR, Chisti Y (2003) Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol Adv 20:491–515
Gupta SK, Kuma NM, Guldhe A, Ansari FA, Rawat I, Nasr M, Bux F (2018) Wastewater to biofuels: comprehensive evaluation of various flocculants on biochemical composition and yield of microalgae. Ecol Eng 117:62–68
Gutiérrez R, Ferre I, García J, Uggetti E (2015) Influence of starch on microalgal biomass recovery, settleability and biogas production. Bioresour Technol 185:341–345
Hansel PA, Riefler RG, Stuart BJ (2014) Efficient flocculation of microalgae for biomass production using cationic starch. Algal Res 5:133–139
Heasman M, Diemar J, O’Connor W, Sushames T, Foulkles L (2000) Development of extended shelf-life microalgae concentrate diets harvested by centrifugation for bivalve mollusks - a summary. Aquac Res 31:637–659
Jiang Y, Zhang W, Wang J, Chen Y, Shen S, Liu T (2013) Utilization of simulated flue gas for cultivation of Scenedesmus dimorphus. Bioresour Technol 128:359–364
Lama S, Muylaert K, Karki TB, Foubert I, Henderson RK, Vandamme D (2016) Flocculation properties of several microalgae and a cyanobacterium species during ferric chloride, chitosan and alkaline flocculation. Bioresour Technol 220:464–470
Larrosa APQ, Camara AS, Pohndorf RS, Rocha SF, Pinto LAA (2018) Physicochemical, biochemical, and thermal properties of Arthrospira (Spirulina) biomass dried in spouted bed at different conditions. J Appl Phycol 30:1019–1029
Lowry OH, Rosebrough NJ, Farr AL, Randall KL (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mattos ER, Singh M, Cabrera ML, Das KC (2015) Enhancement of biomass production in Scenedesmus bijuga high-density culture using weakly absorbed green light. Biomass Bioenergy 81:473–478
Nichols HW, Bold HC (1965) Trichosarcina polymorpha gen. et sp. nov. J Phycol 1:34–38
Noseda MD, Correa, DO, Noseda MED, Oliveira AC, Dominiz, BSN, Rodrigues, JM (2016) Fotobiorreator para cultivo de micro-organismos fotossintetizantes. INPI patent BR1020160294851
Renault F, Sancey B, Badot PM, Crini G (2009) Chitosan for coagulation/flocculation processes - an eco-friendly approach. Eur Polym J 45:1337–1348
Salim S, Bosma R, Vermuë MH, Wijffels RH (2011) Harvesting of microalgae by bio-flocculation. J Appl Phycol 23:849–855
Shubert LE, Wilk-Wozniak E (2003) SEM investigation of several non-motile coccoid green algae isolated from aquatic habitats in Poland. Biol Bratisl 58:459–466
Selesu NFH, de Oliveira TV, Correa DO, Miyawaki B, Mariano AB, Vargas JVC, Vieira RB (2016) Maximum microalgae biomass harvesting via flocculation in large scale photobioreactor cultivation. Can J Chem Eng 94:304–309
Şirin S, Trobajo R, Ibanez C, Salvadó J (2012) Harvesting the microalgae Phaeodactylum tricornutum with polyaluminum chloride, aluminium sulphate, chitosan and alkalinity-induced flocculation. J Appl Phycol 24:1067–1080
Valverde F, Romero-Campero FJ, León R, Guerrero MG, Serrano A (2016) New challenges in microalgae biotechnology. Eur J Protistol 55:95–101
Vandamme D, Foubert I, Meesschaert B, Muylaert K (2010) Flocculation of microalgae using cationic starch. J Appl Phycol 22:525–530
Vieira RB, Vieira PA, Cardoso SL, Ribeiro EJ, Cardoso VL (2012) Sedimentation of mixed cultures using natural coagulants for the treatment of effluents generated in terrestrial fuel distribution terminals. J Hazard Mater 231-232:98–104
Wu K, Liu J, Wu Y, Chen Y, Li Q, Xiao X, Yang M (2014) Pyrolysis characteristics and kinetics of aquatic biomass using thermogravimetric analyzer. Bioresour Technol 163:18–25
Xu Y, Purton S, Baganz F (2013) Chitosan flocculation to aid the harvesting of the microalga Chlorella sorokiniana. Bioresour Technol 129:296–301
Yang R, Li H, Huang M, Yang H, Li A (2016) A review on chitosan-based flocculants and their applications in water treatment. Water Res 95:59–89
Acknowledgments
DOC acknowledges CNPq for a doctoral fellowship. MRD and MDN are Research Members of CNPq. The authors wish to acknowledge the Elizabeth Aidar Microalgae Culture Collection of the Fluminense Federal University (UFF) for the kindly supply of the microalgae used in this work. In addition, we are grateful to the Electronic Microscopy Center of the Paraná Federal University (CME-UFPR) for the SEM analyses.
Funding
This work was financed by the National Council for Scientific and Technological Development, CNPq, Brazil (project nos. 403869/2013-7 and 462414/2014-0).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Corrêa, D.O., Duarte, M.E.R. & Noseda, M.D. Biomass production and harvesting of Desmodesmus subspicatus cultivated in flat plate photobioreactor using chitosan as flocculant agent. J Appl Phycol 31, 857–866 (2019). https://doi.org/10.1007/s10811-018-1586-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-018-1586-z