Abstract
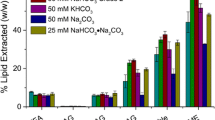
Algal biomass refineries for sustainable transportation fuels, in particular biodiesel, will benefit from algal strain enhancements to improve biomass and lipid productivity. Specifically, the supply of inorganic carbon to microalgal cultures represents an area of great interest due to the potential for improved growth of microalgae and the possibility for incorporation with CO2 mitigation processes. Combinations of bicarbonate (HCO3−) salt addition and application of CO2 to control pH have shown compelling increases in growth rate and lipid productivity of fresh water algae. Here, focus was placed on the marine organism, Nannochloropsis gaditana, to investigate growth and lipid accumulation under various strategies of enhanced inorganic carbon supply. Three gas application strategies were investigated: continuous sparging of atmospheric air, continuous sparging of 5% CO2 during light hours until nitrogen depletion, and continuous sparging of atmospheric air supplemented with 5% CO2 for pH control between 8.0 and 8.3. These gas sparging schemes were combined with addition of low concentrations (5 mM) of sodium bicarbonate at inoculation and high concentration (50 mM) of sodium bicarbonate amendments just prior to nitrogen depletion. The optimum scenario observed for growth of N. gaditana under these inorganic carbon conditions was controlling pH with 5% CO2 on demand, which increased both growth rate and lipid accumulation. Fatty acid methyl esters were primarily comprised of C16:0 (palmitic) and C16:1 (palmitoleic) aliphatic chains. Additionally, the use of high concentration (50 mM) of bicarbonate amendments further improved lipid content (up to 48.6%) under nitrogen deplete conditions when paired with pH-controlled strategies.



Similar content being viewed by others
References
Ahmad AL, Yasin NHM, Derek CJC, Lim JK (2011) Microalgae as a sustainable energy source for biodiesel production: a review. Renew Sust Energy Rev 15:584–593
Amin S (2009) Review on biofuel oil and gas production processes from microalgae. Energy Convers Manag 50:1834–1840
Bhateria R, Dhaka R (2015) Algae as biofuel. Biofuels 5:607–631
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sust Energy Rev 14:557–577
Camacho-Rodríguez J, Cerón-García MC, Fernández-Sevilla JM, Molina-Grima E (2015) The influence of culture conditions on biomass and high value product generation by Nannochloropsis gaditana in aquaculture. Algal Res 11:63–73
Chi Z, O’Fallon JV, Chen S (2011) Bicarbonate produced from carbon capture for algae culture. Trends Biotechnol 29:537–541
Chi Z, Xie Y, Elloy F, Zheng Y, Hu Y, Chen S (2013) Bicarbonate-based integrated carbon capture and algae production system with alkalihalophilic cyanobacterium. Bioresour Technol 133:513–521
Chisti Y (2008) Biodiesel from microalgae beats bioethanol. Trends Biotechnol 26:126–131
Davis R, Aden A, Pienkos PT (2011) Techno-economic analysis of autotrophic microalgae for fuel production. Appl Energy 88:3524–3531
Dong H-P, Williams E, Wang DZ, Xie ZX, Hsia RC, Jenck A, Halden R, Li J, Chen F, Place AR (2013) Responses of Nannochloropsis oceanica IMET1 to long-term nitrogen starvation and recovery. Plant Physiol 162:1110–1126
Fields MW, Hise A, Lohman EJ, Bell T, Gardner RD, Corredor L, Moll K, Peyton BM, Characklis GW, Gerlach R (2014) Sources and resources: importance of nutrients, resource allocation, and ecology in microalgal cultivation for lipid accumulation. Appl Microbiol Biotechnol 98:4805–4816
Gardner RD, Cooksey KE, Mus F, Macur R, Moll K, Eustance E, Carlson RP, Gerlach R, Fields MW, Peyton BM (2012) Use of sodium bicarbonate to stimulate triacylglycerol accumulation in the chlorophyte Scenedesmus sp and the diatom Phaeodactylum tricornutum. J Appl Phycol 24:1311–1320
Gardner RD, Lohman E, Gerlach R, Cooksey KE, Peyton BM (2013a) Comparison of CO2 and bicarbonate as inorganic carbon sources for triacylglycerol and starch accumulation in Chlamydomonas reinhardtii. Biotechnol Bioeng 110:87–96
Gardner RD, Lohman EJ, Cooksey KE, Gerlach R, Peyton BM (2013b) Cellular cycling, carbon utilization, and photosynthetic oxygen production during bicarbonate-induced triacylglycerol accumulation in a Scenedesmus sp. Energies 6:6060–6076
Griffiths MJ, Harrison STL (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507
Guihéneuf F, Stengel DB (2013) LC-PUFA-enriched oil production by microalgae: accumulation of lipid and triacylglycerols containing n-3 LC-PUFA is triggered by nitrogen limitation and inorganic carbon availability in the marine haptophyte Pavlova lutheri. Mar Drugs 11:4246–4266
Hallenbeck PC, Grogger M, Mraz M, Veverka D (2015) The use of Design of Experiments and Response Surface Methodology to optimize biomass and lipid production by the oleaginous marine green alga, Nannochloropsis gaditana in response to light intensity, inoculum size and CO2. Bioresour Technol 184:161–168
Ho S-H, Chen C-Y, Lee D-J, Chang J-S (2011) Perspectives on microalgal CO2-emission mitigation systems—a review. Biotechnol Adv 29:189–198
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Kumar A, Ergas S, Yuan X, Sahu A, Zhang Q, Dewulf J, Malcata FX, van Langenhove H (2010) Enhanced CO2 fixation and biofuel production via microalgae: recent developments and future directions. Trends Biotechnol 28:371–380
Lam MK, Lee KT, Mohamed AR (2012) Current status and challenges on microalgae-based carbon capture. Int J Greenhouse Gas Control 10:456–469
Lardon L, Hélias A, Sialve B, Steyer J-P, Bernard O (2009) Life-cycle assessment of biodiesel production from microalgae. Env Sci Technol 43:6475–6481
Lohman EJ, Gardner RD, Halverson L, Macur RE, Peyton BM, Gerlach R (2013) An efficient and scalable extraction and quantification method for algal derived biofuel. J Microbiol Methods 94:235–244
Lohman EJ, Gardner RD, Pedersen T, Peyton BM, Cooksey KE, Gerlach R (2015) Optimized inorganic carbon regime for enhanced growth and lipid accumulation in Chlorella vulgaris. Biotechnol Biofuels 8:82
Ma Y, Wang Z, Yu C, Yin Y, Zhou G (2014) Evaluation of the potential of 9 Nannochloropsis strains for biodiesel production. Bioresour Technol 167:503–509
Markou G, Vandamme D, Muylaert K (2014) Microalgal and cyanobacterial cultivation: the supply of nutrients. Water Res 65:186–202
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sust Energy Rev 14:217–232
Ördög V, Stirk W, Bálint P, Staden J, Lovász C (2012) Changes in lipid, protein and pigment concentrations in nitrogen-stressed Chlorella minutissima cultures. J Appl Phycol 24:907–914
Pancha I, Chokshi K, Ghosh T, Paliwal C, Maurya R, Mishra S (2015) Bicarbonate supplementation enhanced biofuel production potential as well as nutritional stress mitigation in the microalgae Scenedesmus sp. CCNM 1077. Bioresour Technol 193:315–323
Provasoli L, McLaughlin JJA, Droop MR (1957) The development of artificial media for marine algae. Arch Mikrobiol 25:392–428
Radakovits R, Jinkerson RE, Fuerstenberg SI, Tae H, Settlage RE, Boore JL, Posewitz MC (2012) Draft genome sequence and genetic transformation of the oleaginous alga Nannochloropis gaditana. Nat Commun 3:686.
Ren M, Ogden K (2014) Cultivation of Nannochloropsis gaditana on mixtures of nitrogen sources. Envi Progr Sust Energy 33:551–555
Rocha JM, Garcia JE, Henriques MH (2003) Growth aspects of the marine microalga Nannochloropsis gaditana. Biomol Eng 20:237–242
Sawayama S, Inoue S, Dote Y, Yokoyama S-Y (1995) CO2 fixation and oil production through microalga. Energy Convers Manag 36:729–731
Schenk PM, Thomas-Hall SR, Stephens E, Marx UC, Mussgnug JH, Posten C, Kruse O, Hankamer B (2008) Second generation biofuels: high-efficiency microalgae for biodiesel production. BioEnergy Res 1:20–43
Simionato D, Block MA, La Rocca N, Jouhet J, Maréchal E, Finazzi G, Morosinotto T (2013) The response of Nannochloropsis gaditana to nitrogen starvation includes de novo biosynthesis of triacylglycerols, a decrease of chloroplast galactolipids, and reorganization of the photosynthetic apparatus. Eukaryot Cell 12:665–676
Sydney EB, Sturm W, de Carvalho JC, Thomaz-Soccol V, Larroche C, Pandey A, Soccol CR (2010) Potential carbon dioxide fixation by industrially important microalgae. Bioresour Technol 101:5892–5896
White D, Pagarette A, Rooks P, Ali S (2013) The effect of sodium bicarbonate supplementation on growth and biochemical composition of marine microalgae cultures. J Appl Phycol 25:153–165
Williams PJB, Laurens LML (2010) Microalgae as biodiesel & biomass feedstocks: review & analysis of the biochemistry, energetics & economics. Energy Environ Sci 3:554
Yang Y, Gao K (2003) Effects of CO2 concentrations on the freshwater microalgae, Chlamydomonas reinhardtii, Chlorella pyrenoidosa and Scenedesmus obliquus (Chlorophyta). J Appl Phycol 15:379–389
Acknowledgements
A special thank you to all members of the MSU Algal Biofuels Group for their introspective discourse on algal biofuel-related topics.
Funding
A portion of this research was supported by the U.S. Department of Energy (DOE) Office of Energy Efficiency and Renewable Energy (EERE) Biomass Program under Contract No. DE-EE0005993. Support for TCP was also provided by Church and Dwight Co., Inc. Instrumental support was provided through the Environmental and Biofilm Mass Spectrometry Facility at the College of Engineering (COE), and the Center for Biofilm Engineering (CBE), at Montana State University (MSU).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
A patent entitled “Bicarbonate Trigger for Inducing Lipid Accumulation in Algal Systems” (Pat. No 9,096,875) was co-authored by contributing authors Robert D. Gardner and Brent M. Peyton.
Electronic supplementary material
Electronic Supplementary Fig. 1
Growth [cells mL-1] (a), pH (b), nitrate concentration [mg NO3--N L-1] (c), and total chlorophyll [mg L-1] (d) of cultures of N. gaditana grown using atmospheric air sparge. Air-1: Continuous sparge of atmospheric air (△). Air-2: Continuous sparge of atmospheric air and 50mM NaHCO3 just prior to nitrogen depletion (○). Air-3: Continuous sparge of atmospheric air with 5mM initial NaHCO3 and an additional 50mM NaHCO3 just prior to nitrogen depletion (□). Time of nitrate depletion is indicated in Table 2. Error bars represent ±95% CI (n=3) (JPEG 310 kb)
Electronic Supplementary Fig. 2
Growth [cells mL-1] (a), pH (b), nitrate concentration [mg NO3--N L-1] (c), and total chlorophyll [mg L-1] (d) of cultures of N. gaditana cultured with CO2. CO2-1: Continuous sparge of atmospheric air supplemented with 5% CO2 (v/v) during daylight hours until nitrogen depletion (◇). CO2-2: Continuous sparge of atmospheric air supplemented periodically with 5% CO2 (v/v) to control pH (8.0 to 8.3) (○). Time of nitrate depletion is indicated in Table 2. Error bars represent ±95% CI (n=3) (JPEG 290 kb)
Electronic Supplementary Fig. 3
Growth [cells mL-1] (a), pH (b), nitrate concentration [mg NO3--N L-1] (c), and total chlorophyll [mg L-1] (d) of cultures of N. gaditana cultured with CO2 and bicarbonate. CO2/HCO3-1: pH control (8.0 to 8.3) and supplemented with 50mM NaHCO3 just prior to nitrogen depletion (△). CO2/HCO3-2 and -3: pH control (8.0 to 8.3) with 5mM initial NaHCO3 and an additional 50mM NaHCO3 just prior to nitrogen depletion (○ and ◇). Time of nitrate depletion is indicated in Table 2. Error bars represent ±95% CI (n=3) (JPEG 287 kb)
Electronic Supplementary Fig. 4
Growth [cells mL-1] (a), pH (b), nitrate concentration [mg NO3--N L-1] (c), and total chlorophyll [mg L-1] (d) of cultures of N. gaditana cultured with CO2 and bicarbonate. CO2/HCO3-4: 5% CO2 (v/v) during daylight hours until nitrogen depletion and supplemented with 5mM initial NaHCO3 (□). CO2/HCO3-5: 5% CO2 (v/v) during daylight hours until nitrogen depletion and supplemented with 50mM NaHCO3 just prior to nitrogen depletion (×). Time of nitrate depletion is indicated in Table 2. Error bars represent ±95% CI (n=3) (JPEG 251 kb)
Supplementary Table 1
Estimated alkalinity considering nitrate consumption during each study, initial bicarbonate supplementation, and bicarbonate supplementation at nitrate depletion (DOCX 20.7 kb)
Rights and permissions
About this article
Cite this article
Pedersen, T.C., Gardner, R.D., Gerlach, R. et al. Assessment of Nannochloropsis gaditana growth and lipid accumulation with increased inorganic carbon delivery. J Appl Phycol 30, 2155–2166 (2018). https://doi.org/10.1007/s10811-018-1470-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-018-1470-x