Abstract
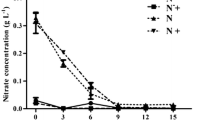
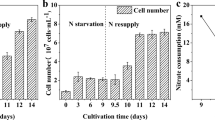
This study investigated the changes in lipid and starch contents, lipid fraction, and lipid profile in the nitrogen-starved Scenedesmus obtusus XJ-15 at different temperatures (17, 25, and 33 °C). The optimal temperature for both growth and lipid accumulation under nitrogen-sufficient condition was found to be 25 °C. However, under nitrogen deprivation, the total and neutral lipids increased with increasing temperature, and achieved the highest lipid content of 47.60 % of dry cell weight and the highest TAG content of 79.66 % of total lipid at 33 °C. In the meantime, the stored cellular starch content decreased with the increasing temperature. Thus, high temperature induced carbon flux from starch toward TAG accumulation in microalgae during nitrogen starvation. In addition, the decreased polar lipids may also serve for TAG synthesis under high temperature, and high temperature further reduced the degree of the fatty acid unsaturation and favored a better biodiesel production. These results suggested that high-temperature stress can be a good strategy for enhancing biofuel production in oleaginous microalgae during nitrogen deficiency.



Similar content being viewed by others
References
Bellou S, Aggelis G (2012) Biochemical activities in Chlorella sp. and Nannochloropsis salina during lipid and sugar synthesis in a lab-scale open pond simulating reactor. J Biotech 164:318–329
Bligh E, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Breuer G, Lamers PP, Martens DE, Draaisma RB, Wijffels RH (2012) The impact of nitrogen starvation on the dynamics of triacylglycerol accumulation in nine microalgae strains. Bioresour Technol 124:217–226
Breuer G, Lamers PP, Martens DE, Draaisma RB, Wijffels RH (2013) Effect of light intensity, pH, and temperature on triacylglycerol (TAG) accumulation induced by nitrogen starvation in Scenedesmus obliquus. Bioresour Technol 143:1–9
Chen CY, Yeh KL, Aisyah R, Lee DJ, Chang JS (2011) Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour Technol 102:71–81
Chen Z, Gong Y, Fang X, Hu H (2012) Scenedesmus sp. NJ-1 isolated from Antarctica: a suitable renewable lipid source for biodiesel production. World J Microbiol Biotech 28:3219–3225
Chi Z, Pyle D, Wen Z, Frear C, Chen S (2007) A laboratory study of producing docosahexaenoic acid from biodiesel-waste glycerol by microalgal fermentation. Process Biochem 42:1537–1545
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process Process Intensif 48:1146–1151
Delrue F, Li-Beisson Y, Setier PA, Sahut C, Roubaud A, Froment AK, Peltier G (2013) Comparison of various microalgae liquid biofuel production pathways based on energetic, economic and environmental criteria. Bioresour Technol 136:205–12
Dragone G, Fernandes BD, Abreu AP, Vicente AA, Teixeira JA (2011) Nutrient limitation as a strategy for increasing starch accumulation in microalgae. Appl Energy 88:3331–3335
Feng P, Deng Z, Hu Z, Wang Z, Fan L (2014) Characterization of Chlorococcum pamirum as a potential biodiesel feedstock. Bioresour Technol 162:115–122
Gardner R, Peters P, Peyton B, Cooksey KE (2010) Medium pH and nitrate concentration effects on accumulation of triacylglycerol in two members of the chlorophyta. J Appl Phycol 23:1005–1016
Gardner RD, Lohman E, Gerlach R, Cooksey KE, Peyton BM (2013) Comparison of CO2 and bicarbonate as inorganic carbon sources for triacylglycerol and starch accumulation in Chlamydomonas reinhardtii. Biotechnol Bioeng 110:87–96
Goñi I, Garcia-Alonso A, Saura-Calixto F (1997) A starch hydrolysis procedure to estimate glycemic index. Nutr Res 17:427–437
Griffiths MJ, Harrison STL (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507
Griffiths MJ, Hille RP, Harrison STL (2011) Lipid productivity, settling potential and fatty acid profile of 11 microalgal species grown under nitrogen replete and limited conditions. J Appl Phycol 24:989–1001
Griffiths MJ, van Hille RP, Harrison ST (2014) The effect of nitrogen limitation on lipid productivity and cell composition in Chlorella vulgaris. Appl Microbiol Biotechnol 98:2345–2356
Guschina IA, Harwood JL (2006) Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res 45:160–186
Hamid Rismani-Yazdi BZH, Carol Hsin, Jordan Peccia (2012) Transcriptomic analysis of the oleaginous microalga Neochloris oleoabundans reveals metabolic insights into triacylglyceride accumulation. Biotechnol Biofuels 5:74
Ho SH, Chen CY, Chang JS (2012) Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour Technol 113:244–52
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
James GO, Hocart CH, Hillier W, Price GD, Djordjevic MA (2013) Temperature modulation of fatty acid profiles for biofuel production in nitrogen deprived Chlamydomonas reinhardtii. Bioresour Technol 127:441–447
Klein U, Betz A (1978) Fermentative metabolism of hydrogen-evolving Chlamydomonas moewusii. Plant Physiol 61:953–956
Knothe G (2011) A technical evaluation of biodiesel from vegetable oils vs. algae. Will algae-derived biodiesel perform? Green Chem 13:3048–3065
Li Y, Horsman M, Wang B, Wu N, Lan CQ (2008) Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biotechnol 81:629–636
Li Y, Han D, Hu G, Sommerfeld M, Hu Q (2010) Inhibition of starch synthesis results in overproduction of lipids in Chlamydomonas reinhardtii. Biotechnol Bioeng 107:258–268
Li X, Hu HY, Zhang YP (2011a) Growth and lipid accumulation properties of a freshwater microalga Scenedesmus sp. under different cultivation temperature. Bioresour Technol 102:3098–102
Li Y, Han D, Sommerfeld M, Hu Q (2011b) Photosynthetic carbon partitioning and lipid production in the oleaginous microalga Pseudochlorococcum sp. (Chlorophyceae) under nitrogen-limited conditions. Bioresour Technol 102:123–9
Mandal S, Mallick N (2009) Microalga Scenedesmus obliquus as a potential source for biodiesel production. Appl Microbiol Biotechnol 84:281–291
Markou G, Angelidaki I, Georgakakis D (2012) Microalgal carbohydrates: an overview of the f actors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl Microbiol Biotechnol 96:631–645
Münkel R, Schmid-Staiger U, Werner A, Hirth T (2013) Optimization of outdoor cultivation in flat panel airlift reactors for lipid production by Chlorella vulgaris. Biotechnol Bioeng 110:2882–2893
Mus F, Toussaint JP, Cooksey KE, Fields MW, Gerlach R, Peyton BM, Carlson RP (2013) Physiological and molecular analysis of carbon source supplementation and pH stress-induced lipid accumulation in the marine diatom Phaeodactylum tricornutum. Appl Microbiol Biotechnol 97:3625–42
Nascimento I, Marques S, Cabanelas I, Pereira S, Druzian J, Souza C, Vich D, Carvalho G, Nascimento M (2013) Screening microalgae strains for biodiesel production: lipid productivity and estimation of fuel quality based on fatty acids profiles as selective criteria. Bioenergy Res 6:1–13
Pan YY, Wang ST, Chuang LT, Chang YW, Chen CN (2011) Isolation of thermo-tolerant and high lipid content green microalgae: oil accumulation is predominantly controlled by photosystem efficiency during stress treatments in Desmodesmus. Bioresour Technol 102:10510–10517
Pribyl P, Cepak V, Zachleder V (2012) Production of lipids in 10 strains of Chlorella and Parachlorella, and enhanced lipid productivity in Chlorella vulgaris. Appl Microbiol Biotechnol 94:549–561
Rai LC, Mallick N, Singh JB, Kumar HD (1991) Physiological and biochemical characteristics of a copper tolerant and a wild type strain of Anabaena doliolum under copper stress. J Plant Physiol 138:68–74
Rodolfi L, Chini Zittelli G, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–12
Sharma KK, Schuhmann H, Schenk PM (2012) High lipid induction in microalgae for biodiesel production. Energies 5:1532–1553
Su CH, Chien LJ, Gomes J, Lin YS, Yu YK, Liou JS, Syu RJ (2010) Factors affecting lipid accumulation by Nannochloropsis oculata in a two-stage cultivation process. J Appl Phycol 23:903–908
Takeshita T, Ota S, Yamazaki T, Hirata A, Zachleder V, Kawano S (2014) Starch and lipid accumulation in eight strains of six Chlorella species under comparatively high light intensity and aeration culture conditions. Bioresour Technol 158:127–134
Tang Z (1999) Experimental handbook of modern plant physiology. Science Press, Beijing, pp 127–128
Venkata Subhash G, Rohit MV, Devi MP, Swamy YV, Venkata Mohan S (2014) Temperature induced stress influence on biodiesel productivity during mixotrophic microalgae cultivation with wastewater. Bioresour Technol 169:789–793
Wan MX, Wang RM, Xia JL, Rosenberg JN, Nie ZY, Kobayashi N, Oyler GA, Betenbaugh MJ (2012) Physiological evaluation of a new Chlorella sorokiniana isolate for its biomass production and lipid accumulation in photoautotrophic and heterotrophic cultures. Biotechnol Bioeng 109:1958–1964
Wattebled F, Ral JP, Dauvillée D, Myers AM, James MG, Schlichting R, Giersch C, Ball SG, D’Hulst C (2003) STA11, a Chlamydomonas reinhardtii locus required for normal starch granule biogenesis, encodes disproportionating enzyme. Further evidence for a function of α-1, 4 glucanotransferases during starch granule biosynthesis in green algae. Plant Physiol 132:137–145
Xia L, Ge H, Zhou X, Zhang D, Hu C (2013) Photoautotrophic outdoor two-stage cultivation for oleaginous microalgae Scenedesmus obtusus XJ-15. Bioresour Technol 144:261–267
Xia L, Song S, He Q, Yang H, Hu C (2014a) Selection of microalgae for biodiesel production in a scalable outdoor photobioreactor in north China. Bioresour Technol 174:274–280
Xia L, Yang H, He Q, Hu C (2014b) Physiological responses of freshwater oleaginous microalgae Desmodesmus sp. NMX451 under nitrogen deficiency and alkaline pH-induced lipid accumulation. J Appl Phycol doi:10.1007/s10811-014-0371-x
Yao CH, Ai JN, Cao XP, Xue S (2013) Characterization of cell growth and starch production in the marine green microalga Tetraselmis subcordiformis under extracellular phosphorus-deprived and sequentially phosphorus-replete conditions. Appl Microbiol Biotechnol 97:6099–6110
Zachleder V, Brányiková I (2014) Starch overproduction by means of algae. In: Bajpai R, Prokop A, Zappi M (eds) Algal biorefineries. Springer, Netherlands, pp 217–240
Zhu S, Huang W, Xu J, Wang Z, Xu J, Yuan Z (2014) Metabolic changes of starch and lipid triggered by nitrogen starvation in the microalga Chlorella zofingiensis. Bioresour Technol 152:292–298
Acknowledgments
This work was financially supported by the National 863 program (2013AA065804), International Partner Program of Innovation Team (Chinese Academy of Sciences), Platform Construction of Oleaginous Microalgae (Institute of Hydrobiology, CAS of China).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xia, L., Song, S. & Hu, C. High temperature enhances lipid accumulation in nitrogen-deprived Scenedesmus obtusus XJ-15. J Appl Phycol 28, 831–837 (2016). https://doi.org/10.1007/s10811-015-0636-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0636-z