Abstract
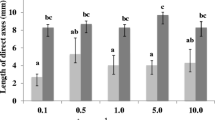
Spindle inhibitors and phytoregulators are substances largely used to improve the growth of plants with commercial interest. This study analyzes the effects of colchicine (Ch) and oryzalin (Oz), two spindle inhibitors, on the survival and growth rates of Kappaphycus alvarezii micropropagules when combined in cultures with such phytoregulators as indole-3-acetic acid (IAA), kinetin (K), and spermine (S), aiming to improve mass cultivation under laboratory conditions. Explants were first cultured in treatments of colchicine and oryzalin, separately or in combination, for 2 weeks. Afterwards, they were cultured in sterilized seawater enriched with 50 % von Stosch solution. All explants produced upright axes from the cut or lateral region. Treatment with 1.0 mg L−1 oryzalin significantly increased the formation of upright axes (4.89 ± 1.60 upright axes explant−1) compared to control (1.33 ± 0.16 upright axes explant−1). Explants treated with oryzalin alone or in combination with IAA or kinetin showed a significantly higher production of upright axes, while those treated with oryzalin in combination with spermine or other phytoregulators (IAA and K; IAA and S; K and S; IAA, K, and S) showed a significant increase of growth rates, ranging 3.47 ± 0.27 % day−1in control to 4.98 ± 0.47 % day−1in the IAA:S + Oz treatment. Results showed that oryzalin in combination with phytoregulators, such as IAA, spermine, or kinetin, affects micropropagule production and growth, thereby improving the mass cultivation of K. alvarezii but without affecting the survival rates of explants.








Similar content being viewed by others
References
Andersen RA (2005) Algal culturing techniques. Elsevier, Amsterdam
Azanza-Corrales R, Dawes C (1989) Wound healing in cultured Eucheuma alvarezii var. tambalang Doty. Bot Mar 32:229–234
Barandalla L, Ritter E, Galarreta JIR (2006) Oryzalin treatment of potato diploids yields tetraploid and chimeric plants from which euploids could be derived by callus induction. Potato Res 49:143–154
Collantes G, Melo C, Candia A (2004) Micropropagation by explants of Gracilaria chilensis Bird, McLachlan and Oliveira. J Appl Phycol 16:203–213
Dawes CJ, Koch EW (1991) Branch, micropropagule and tissue culture of the red algae Eucheuma denticulatum and Kappaphycus alvarezii farmed in the Philippines. J Appl Phycol 3:247–257
Dawes CJ, Trono GC Jr, Lluisma AO (1993) Clonal propagation of Eucheuma denticulatum and Kappaphycus alvarezii for Philippine seaweed farms. Hydrobiologia 260/261:379–383
Edwards P (1970) Illustrated guide to the seaweeds and sea grasses in the vicinity of Porto Aransas, Texas. Contributions in Marine Science 15:1-228. In: Aveal K, Ferrario ME, Oliveira EC, Sar E (1995) Manual e métodos ficológicos. Ediciones da Universidade de Concepción
Glowacka K, Jezowski S, Kaczamarek Z (2010) In vitro induction of polyploidy by colchicine treatment of shoots and preliminary characterisation of induced polyploids in two Miscanthus species. Ind Crop Prod 32:88–96
Hayashi L, Yokoya NS, Kikuchi DM, Oliveira EC (2008) Callus induction and micropropagation improved by colchicine and phytoregulators in Kappaphycus alvarezii (Rhodophyta, Solieriaceae). J Appl Phycol 20:653–659
Hugdahl JD, Morejohn LC (1993) Rapid and reversible high-affinity binding of the dinitroaniline herbicide oryzalin to tubulin from Zea mays L. Plant Physiol 102:725–740
Hurtado AQ, Yunque DA (2009) Use of Acadian marine plant extract powder from Ascophyllum nodosum in tissue culture of Kappaphycus varieties. J Appl Phycol 21:633–639
Ivanovskii YA, Kulepanov VN (2008) Initiation of gametophytic callus and somatic embryogenesis in Laminaria japonica (Phaeophyta) exposed to mutagenic agents. Russ J Mar Biol 34:325–328
Karp A (1995) Somaclonal variation as a tool for crop improvement. Euphytica 85:295–302
Lignell A, Pedersén M (1989) Agar composition as function of morphology and growth rate. Studies on some morphological strains of Gracilaria secundata and Gracilaria verrucosa (Rhodophyta). Bot Mar 32:219–227
Marián FR, García-Jiménez P, Robaina RR (2000) Polyamines in marine macroalgae: levels of putrecine, spermidine and spermine in thalli and changes in their concentration during glycerol-induced cell growth in vitro. Physiol Plant 110:530–534
Muñoz J, Cahue-López AC, Patiño R, Robledo D (2006) Use of plant growth regulators in micropropagation of Kappaphycus alvarezii (Doty) in airlift bioreactors. J Appl Phycol 18:209–218
Paula EJ, Pereira RTL, Ohno M (1999) Strain selection in Kappaphycus alvarezii var. alvarezii (Soleriaceae, Rhodophyta) using tetraspore progeny. J Appl Phycol 11:111–121
Stirk WA, Novák O, Strnad M, van Staden J (2003) Cytokinins in macroalgae. Plant Growth Regul 41:13–24
Tarakhovskaya ER, Maslov YI, Shishova MF (2007) Phytohormones in algae. Russ J Plant Physl 54:163–170
Tilden PM, Kenneth WL (2011) In vitro polyploid induction of orchids using oryzalin. Sci Hort 130:314–319
Titlyanov EA, Titlyanova TV (2010) Seaweed cultivation: methods and problems. Russ J Mar Biol 36:227–242
Wacker I, Quader H, Schnepf E (1987) Influence of the herbicide oryzalin on cytoskeleton and growth of Funaria hygrometrica protonemata. Protoplasma 142:55–67
Yokoya NS (2000) Apical callus formation and plant regeneration controlled by plant growth regulators on axenic culture of the red alga Gracilariopsis tenuifrons (Gracilariales, Rhodophyta). Phycol Res 48:132–142
Yokoya NS, Stirk WA, van Staden J, Novák O, Turecková V, Pěnčík A, Strmad M (2010) Endogenous cytokinins, auxins, and abscisic acid in red algae from Brazil. J Phycol 46:1198–1205
Yunque DAT, Tibubos KR, Hurtado AQ, Critchley AT (2011) Optimization of culture conditions for tissue culture production of young plantlets of carrageenophyte Kappaphycus. J Appl Phycol 23:433–438
Zitta CS, Rover T, Hayashi L, Bouzon ZL (2013) Callus ontogeny of the Kappaphycus alvarezii (Rhodophyta, Gigartinales) brown tetrasporophyte strain. J Appl Phycol 25:615–629
Acknowledgments
This study received financial support from the Coordenação de Aperfeiçoamento de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Neves, F.A.S., Simioni, C., Bouzon, Z.L. et al. Effects of spindle inhibitors and phytoregulators on the micropropagation of Kappaphycus alvarezii (Rhodophyta, Gigartinales). J Appl Phycol 27, 437–445 (2015). https://doi.org/10.1007/s10811-014-0309-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0309-3