Abstract
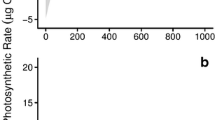
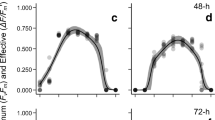
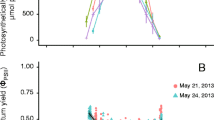
The photosynthetic performance of two Indonesian carrageenophytes (Solieriaceae), Eucheuma denticulatum and Kappaphycus sp. (so-called Sumba strain), was investigated under a variety of temperature and light conditions regarding their mariculture performance. A pulse amplitude modulated-chlorophyll fluorometer (Diving-PAM) was used to generate rapid light curves (RLCs) to provide estimates of the relative electron transport rates (rETR) for over 10 temperatures (i.e., from 16 to 34 °C) and at nine levels of photosynthetic active radiation, which ranged from 0 to 1,000 μmol photons m−2 s−1. Underwater irradiance in a cultivation area was also measured at the collection site in South Sulawesi, Indonesia. The initial slope (α), photoinhibition coefficient (β), and the coefficient of maximum photosynthesis assuming no photoinhibition (γ) was calculated by fitting the RLCs to a nonlinear model of the form \( {\text{rETR}} = \gamma \left( {1 - \exp \left( { - \frac{\alpha }{\gamma }{\text{PAR}}} \right)} \right)\left( {\exp \left( { - \frac{\beta }{\gamma }{\text{PAR}}} \right)} \right) \) using a two-level hierarchical Bayesian model. The experiments revealed that E. denticulatum and Kappaphycus sp. required temperatures ranging from 23 to 32 °C and 22 to 33 °C to maintain high rates of photosynthetic activity, respectively. Clearly, both species appear to be well-adapted to the natural light and temperature conditions at the cultivation site, and we expect the results of this study will be useful for the design and sustainable management of similar mariculture activity.




Similar content being viewed by others
References
Aguirre-von-Wobeser E, Figueroa FL, Cabello-Pasini A (2001) Photosynthesis and growth of red and green morphotypes of Kappaphycus alvarezii (Rhodophyta) from the Philippines. Mar Biol 138:679–686
Aldea M, Frank TD, DeLucia EH (2006) A method for quantitative analysis of spatially variable physiological processes across leaf surface. Photosynth Res 90:161–172
Amin M, Rumayar TP, Femmi NF, Keemur D, Suwitra IK (2008) The assessment of seaweed (Eucheuma cottonii) growing practice of different systems and planting seasons in Bangkep Regency Central Sulawesi. Indonesian J Agricul Sci 1:132–139
Anthony KRN, Ridd PV, Orpin AR, Larcombe P, Lough J (2004) Temporal variation of light availability in coastal benthic habitats. Effects of clouds, turbidity, and tides. Limnol Oceanogr 49:2201–2211
Beer S, Björk M (2000) Measuring rates of photosynthesis of two tropical seagrasses by pulse amplitude modulated (PAM) fluorometry. Aquatic Bot 66:69–76
Beer S, Vilenkin B, Weill A, Veste M, Susell L, Eshel A (1998) Measuring photosynthetic rates in seagrasses by pulse amplitude modulated (PAM) fluorometry. Mar Ecol Prog Ser 174:293–300
Bejarano RI (2006) Relationships between reef fish communities, water and habitat quality on coral reefs. M.S. thesis, University of Puerto Rico, Mayagüez
Bhujel RC (2008) Statistics for aquaculture. Wiley-Blackwell, Iowa
Bixler HJ, Porse H (2011) A decade of change in seaweed hydrocolloids industry. J Appl Phycol 23:321–335
Cosgrove J, Borowitzka MA (2011) Chlorophyll fluorescence terminology: an introduction. In: Suggett DJ, Prášil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic sciences: methods and developments. Developments in Applied Phycology 4. Springer, Dordrecht, pp 1–17
Dawes CJ, Lluisma AO, Trono GC (1994) Laboratory and field growth studies of commercial strains of Eucheuma denticulatum and Kappaphycus alvarezii in the Philiphines. J Appl Phycol 6:21–24
Enriquez S, Borowitzka MA (2011) The use of the fluorescence signal in studies of seagrass and macroalgae. In: Suggett DJ, Prášil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic sciences: methods and developments. Developments in Applied Phycology 4. Springer, Dordrecht, pp 187–208
Gevaert F, Creach A, Davoult D, Holl AC, Seuront L, Lemoine Y (2002) Photo-inhibition and seasonal photosynthetic performance of the seaweed Laminaria saccharina during a simulated tidal cycle: chlorophyll fluorescence measurements and pigment analysis. Plant Cell Environ 25:859–872
Glynn PW, Welington GM, Wells JW (1983) Corals and coral reefs of the Galápagos Islands. University of California Press, London
Huppertz K, Hanelt D, Nultsch W (1990) Photoinhibition of photosynthesis in the marine brown algae Fucus serratus as studied in field experiments. Mar Ecol Prog Ser 66:175–182
Kuster A, Pohl K, Altenburger R (2007) A fluorescence-based bioassay for aquatic macrophytes and its suitability for effect analysis of non-photosystem II inhibitors. Env Sci Pollut Res 14:377–383
Lideman, Nishihara GN, Noro T, Terada R (2011) In vitro growth and photosynthesis of three edible seaweeds, Betaphycus gelatinus, Eucheuma serra and Meristotheca papulosa (Solieriaceae, Rhodophyta). Aquaculture Sci 59:563–571
Nonji A (1993) Indonesian Ocean. Djambatan, Jakarta
Ohno M, Largo DB, Ikumoto T (1994) Growth rate, carrageenan yield and gel properties of cultured kappa-carrageenan producing red alga Kappaphycus alvarezii (Doty) Doty in the subtropical waters of Shikoku. Japan J Appl Phycol 6:1–5
Platt T, Gallegos CL, Harrison WG (1980) Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J Mar Res 38:687–701
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org
Ralph PJ, Gademann R, Dennison WC (1998) In situ seagrass photosynthesis measured using a submersible, pulse-amplitude modulated fluorometer. Mar Biol 32:367–373
Ralph PJ, Schreiber U, Gademann R, Kuhl M, Larkum AWD (2006) Coral photobiology studied with a new imaging pulse amplitude modulated fluorometer. J Phycol 41:335–342
Renger G, Schreiber U (1986) Practical applications of fluorometric methods to algae and higher plant research. In: Govindjee, Amesz J, Fork DD (eds) Light emission by plants and bacteria. Academic Press, Orlando
Schreiber U, Hormann H, Neubauer C, Klughammer C (1995) Assessment of photosystem II, photochemical quantum yield by chlorophyll fluorescence quenching analysis. Aust J Plant Physiol 22:209–220
Soegiarto A, Birowo S, Sukarno (1976) Map of Indonesian Ocean. Indonesian Science Institute
Thomas A, O’Hara B, Ligges U, Sturtz S (2006) Making BUGS Open. R News 6:12–17
Tomascik T, Mah AJ, Nonji A, Moosa MK (1997) The ecology of Indonesian seas. Part Two. Periplus Editions (HK) Ltd, Singapore
Waaland JR (1981) Commercial utilization. In: Loban CS, Wynne MJ (eds) The biology of seaweeds. University of California Press, Berkley, pp 726–741
Wobeser EA, Figueroa FL, Cabello-Pasini A (2001) Photosynthesis and growth of red and green morphotypes of Kappaphycus alvarezii (Rhodophyta) from the Philippines. Mar Biol 138:679–686
Acknowledgments
This research was sponsored in part by Grant-in-Aid for Scientific Research (#22510033) from the Japanese Ministry of Education, Culture, Sport, and Technology (RT), and the Nagasaki University Strategy for Fostering Young Scientist with funding provided by the Special Coordination Funds for Promoting Science and Technology of Ministry of Education, Culture, Sport, Science and Technology (GNN). We would like to expresses our thanks to Director of Central for Brackish-water Aquaculture Development Center, for their kind contributions to the collections of samples and measurement of underwater irradiance in their cooperating farming area, South Sulawesi, Indonesia. We also thank Dr. Michael A. Borowitzka, and two reviewers for their valuable suggestion to improve the manuscript. This research was also the part of dissertation submitted by the first author in partial fulfillment of the Ph.D. degree.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lideman, Nishihara, G.N., Noro, T. et al. Effect of temperature and light on the photosynthesis as measured by chlorophyll fluorescence of cultured Eucheuma denticulatum and Kappaphycus sp. (Sumba strain) from Indonesia. J Appl Phycol 25, 399–406 (2013). https://doi.org/10.1007/s10811-012-9874-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-012-9874-5