Abstract
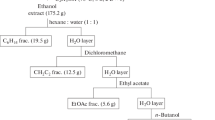
A microwave-assisted extraction method has been developed for the extraction of phenolic compounds from the green alga, Caulerpa racemosa. An L18(3)5 orthogonal experimental array was designed to optimize the extraction conditions. Total phenolic content was determined by Folin–Ciocalteu method. Under the optimized conditions (microwave power, 200 W; ethanol concentration, 60%; extraction time, 40 min; extraction temperature, 50°C; solvent-to-material ratio, 40 mL g−1), the maximum total phenolic content reached 67.89 ± 3.88 mg 100 g−1 dried sample. The crude ethanolic extract was further purified by liquid–liquid partition to afford two fractions, of which the ethyl acetate-soluble fraction (EAF) exhibited the strongest antioxidant activity in the hydroxyl and 2,2-diphenyl-1-picrylhydrazyl radical scavenging assays and reducing power. EAF was further divided into four subfractions, designated as EAF1 to EAF4, by silica gel vacuum liquid chromatography. The antioxidant capacity of the subfractions was in the following order: EAF1>EAF2>EAF4>EAF3. The results of IR spectral and HPLC analysis, including the research on the correlation between antioxidant capacity and total phenolic content, suggested that phenolic compounds of medium polarity were the major contributors to the antioxidative activity of C. racemosa. The present findings might contribute to a rational basis for the use of phenolic-rich fractions and subfractions as natural antioxidants in different food/pharmaceutical products.







Similar content being viewed by others
References
Agarwal R, Tandon P, Gupta VD (2006) Phonon dispersion in poly (dimethylsilane). J Organometallic Chem 691:2902–2908
Ballard TS, Mallikarjunan P, Zhou K, O’Keefe S (2010) Microwave-assisted extraction of phenolic antioxidant compounds from peanut skins. Food Chem 120:1185–1192
Bekçi Z, Seki Y, Cavas L (2009) Removal of malachite green by using an invasive marine alga Caulerpa racemosa var. cylindracea. J Hazard Mater 161:1454–1460
Bilska A, Wlodek L (2005) Lipoic acid—the drug of the future? Pharmacol Rep 57:570–577
Bryngelsson S, Dimberg LH, Kamal-Eldin A (2002) Effects of commercial processing on levels of antioxidants in oats (Avena sativa L.). J Agric Food Chem 50:1890–1896
Bursali EA, Cavas L, Seki Y, Bozkurt SS, Mt Y (2009) Sorption of boron by invasive marine seaweed: Caulerpa racemosa var. cylindracea. Chem Eng J 150:385–390
Cengiz S, Cavas L (2008) Removal of methylene blue by invasive marine seaweed: Caulerpa racemosa var. cylindracea. Biores Tech 99:2357–2363
Chen CH, Pearson AM, Gray JI (1992) Effects of synthetic antioxidants (BHA, BHT and PG) on the mutagenicity of IQ-like compounds. Food Chem 43:177–183
Chirinos R, Betalleluz-Pallardel I, Huamán A, Arbizu C, Pedreschi R, Campos D (2009) HPLC-DAD characterisation of phenolic compounds from Andean oca (Oxalis tuberosa Mol.) tubers and their contribution to the antioxidant capacity. Food Chem 113:1243–1251
Dorman HJD, Kosar M, Kahlos K, Holm Y, Hiltunen R (2003) Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties, and cultivars. J Agric Food Chem 51:4563–4569
Duan XJ, Zhang WW, Li XM, Wang BG (2006) Evaluation of antioxidant property of extract and fractions obtained from a red alga, Polysiphonia urceolata. Food Chem 95:37–43
Ganesan P, Kumar CS, Bhaskar N (2008) Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Biores Technol 99:2717–2723
Ghosh P, Adhikari U, Ghosal PK, Pujol CA, Carlucci MJ, Damonte EB, Ray B (2004) In vitro anti-herpetic activity of sulfated polysaccharide fractions from Caulerpa racemosa. Phytochemistry 65:3151–3157
Hayat K, Hussain S, Abbas S, Farooq U, Ding BM, Xia SQ, Jia CQ, Zhang XM, Xia WS (2009) Optimized microwave-assisted extraction of phenolic acids from citrus mandarin peels and evaluation of antioxidant activity in vitro. Sep Purif Technol 70:63–70
Jin M, Cai YX, Li JR, Zhao H (1996) 1,10-Phenanthroline–Fe2+ oxidative assay of hydroxyl radical produced by H2O2/Fe2+. Prog Biochem Biophys 23:553–555
Li HB, Cheng KW, Wong CC, Fan KW, Chen F, Jiang Y (2007) Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem 102:771–776
López A, Rico M, Rivero A, Suarez M, Suárez de Tangil M (2011) The effects of solvents on the phenolic contents and antioxidant activity of Stypocaulon scoparium algae extracts. Food Chem 125:1104–1109
Lu X, Webb M, Talbott M, Van Eenennaam J, Palumbo A, Linares-Casenave J, Doroshov S, Struffenegger P, Rasco B (2010) Distinguishing ovarian maturity of farmed white sturgeon (Acipenser transmontanus) by Fourier transform infrared spectroscopy: a potential tool for caviar production management. J Agric Food Chem 58:4056–4064
Luo HY, Wang B, Yu CG, Qu YL, Su CL (2010a) Evaluation of antioxidant activities of five selected brown seaweeds from China. J Med Plant Res 4:2557–2565
Luo HY, Wang B, Yu CG, Xu YF (2010b) Optimization of microwave-assisted extraction of polyphenols from Enteromorpha prolifra by orthogonal test. Chin Herb Med 2:321–325
Maheshwari DT, Yogendra Kumar MS, Verma SK, Singh VK, Singh SN (2011) Antioxidant and hepatoprotective activities of phenolic rich fraction of Seabuckthorn (Hippophae rhamnoides L.) leaves. Food Chem Toxicol 49:2422–2428
Mannan H, Mohammad AF, Najmeh M, Neda S, Mohammad RO, Nastaran NV (2010) Evaluation of antioxidant properties and total phenolic contents of some strains of microalgae. J Appl Phycol 22:43–50
Mao WJ, Zang XX, Li Y, Zhang HJ (2006) Sulfated polysaccharides from marine green algae Ulva conglobata and their anticoagulant activity. J Appl Phycol 18:9–14
Marioda AA, Ibrahima RM, Ismaila M, Ismaila N (2009) Antioxidant activity and phenolic content of phenolic rich fractions obtained from black cumin (Nigella sativa) seedcake. Food Chem 116:306–312
Matanjun P, Mohamed S, Mustapha NM, Muhammad K, Ming CH (2008) Antioxidant activities and phenolics content of eight species of seaweeds from north Borneo. J Appl Phycol 20:367–373
Peinado J, Lopez de Lerma N, Moreno J, Peinado RA (2009) Antioxidant activity of different phenolics fractions isolated in must from Pedro Ximenez grapes at different stages of the off-vine drying process. Food Chem 114:1050–1055
Proestos C, Komaitis M (2008) Application of microwave-assisted extraction to the fast extraction of plant phenolic compounds. LWT- Food Sci Technol 41:652–659
Rawat S, Bhatt ID, Rawal RS (2011) Total phenolic compounds and antioxidant potential of Hedychium spicatum Buch Ham. ex D. Don in west Himalaya, India. J Food Compos Anal 24:574–579
Rodrigo R, Guichard C, Charles R (2007) Clinical pharmacology and therapeutic use of antioxidant vitamins. Fundam Clin Pharmacol 21:111–127
Saito M, Kawai M, Hagino H, Okada J, Yamamoto K, Hayashida M, Ikeda T (2002) Antihypertensive effect of Nori-peptides derived from red alga Porphyra yezoensis in hypertensive patients. Am J Hypertens 15:210
Schulz H, Baranska M (2007) Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib Spectrosc 43:13–25
Sheih IC, Wu TK, Fang TJ (2009a) Antioxidant properties of a new antioxidative peptide from algae protein waste hydrolysate in different oxidation systems. Biores Technol 100:3419–3425
Sheih IC, Wu TK, Fang TJ (2009b) Isolation and characterisation of a novel angiotensin I-converting enzyme (ACE) inhibitory peptide from the algae protein waste. Food Chem 115:279–284
Shi C, Xu MJ, Bayer M, Deng ZW, Kubbutat HG, Waejen W, Proksch P, Lin WH (2010) Phenolic compounds and their anti-oxidative properties and protein kinase inhibition from the Chinese mangrove plant Laguncularia racemosa. Phytochemistry 71:435–442
Soares AA, Marques de Souza CG, Daniel FM, Ferrari GP, Gomes da Costa SM, Peralta RM (2009) Antioxidant activity and total phenolic content of Agaricus brasiliensis (Agaricus blazei Murril) in two stages of maturity. Food Chem 112:775–781
Sutivisedsak N, Cheng HN, Willett JL, Lesch WC, Tangsrud RR, Biswas A (2010) Microwave-assisted extraction of phenolics from bean (Phaseolus vulgaris L.). Food Res Int 43:516–519
Tannin-Spitz T, Bergman M, van-Moppes D, Grossman S, Arad S (2005) Antioxidant activity of the polysaccharide of the red microalga Porphyridium sp. J Appl Phycol 17:215–222
Tasioula-Margari M, Okogeri O (2001) Simultaneous determination of phenolic compounds and tocopherols in virgin olive oil using HPLC and UV detection. Food Chem 74:377–383
Tsubaki S, Sakamoto M, Azuma J (2010) Microwave-assisted extraction of phenolic compounds from tea residues under autohydrolytic conditions. Food Chem 123:1255–1258
Verlaque M, Durand C, Huisman JM, Boudouresque CF, Parco Y (2003) On the identity and origin of the Mediterranean invasive Caulerpa racemosa (Caulerpales, Chlorophyta). Eur J Phycol 38:325–339
Victor VM, Rocha M (2007) Targeting antioxidants to mitochondria: a potential new therapeutic strategy for cardiovascular diseases. Curr Pharm Des 13:845–863
Wang BG, Zhang WW, Duan XJ, Li XM (2009) In vitro antioxidative activities of extract and semi-purified fractions of the marine red alga, Rhodomela confervoides (Rhodomelaceae). Food Chem 113:1101–1105
Wang B, Yu CG, Luo HY, Qu YL, Yang LY (2010) Studies on the preparation and antioxidant properties of enzymatic hydrolysate from Dasyatis akajei by papain. Food Sci Technol Int 10:113–118
Williams GM, Iatropoulos MJ, Whysner J (1999) Safety assessment to butylated hydroxyanisol and butylated hydroxytoluene as antioxidant food additives. Food Chem Toxicol 37:1027–1038
Yu PZ, Zhang QB, Li N, Xu ZH, Wang YM, Li ZE (2003) Polysaccharides from Ulva pertusa (Chlorophyta) and preliminary studies on their antihyperlipidemia activity. J Appl Phycol 15:21–27
Zhang B, Yang RY, Liu CZ (2008) Microwave-assisted extraction of chlorogenic acid from flower buds of Lonicera japonica Thunb. Sep Purif Technol 62:480–483
Acknowledgments
This work was financed by National Natural Science Foundation of China (No.81001393), the State-level Spark Program (2010GA700088), and Zhejiang Provincial Natural Science Foundation (No.Y2110636). The authors thank Dr. Changfeng Chi for her critical reading and corrections of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Z., Wang, B., Zhang, Q. et al. Preparation and antioxidant property of extract and semipurified fractions of Caulerpa racemosa . J Appl Phycol 24, 1527–1536 (2012). https://doi.org/10.1007/s10811-012-9813-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-012-9813-5