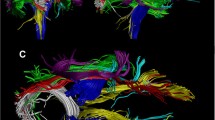
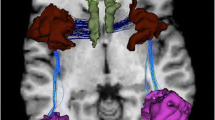
Abstract
Mirror neuron system (MNS) dysfunctions might underlie deficits in autism spectrum disorders (ASD). Diffusion tensor imaging based probabilistic tractography was conducted in 15 adult ASD patients and 13 matched, healthy controls. Fractional anisotropy (FA) was quantified to assess group differences in tract-related white matter microstructure of both the classical MNS route (mediating “emulation”) and the alternative temporo-frontal route (mediating “mimicry”). Multiple linear regression was used to investigate structure–function relationships between MNS connections and ASD symptom severity. There were no significant group differences in tract-related FA indicating an intact classical MNS in ASD. Direct temporo-frontal connections could not be reconstructed challengeing the concept of multiple routes for imitation. Tract-related FA of right-hemispheric parieto-frontal connections was negatively related to autism symptom severity.


Similar content being viewed by others
References
Ameis, S. H., & Catani, M. (2015). Altered white matter connectivity as a neural substrate for social impairment in autism spectrum disorder. Cortex; A Journal Devoted to the Study of the Nervous System and Behavior, 62, 158–181. doi:10.1016/j.cortex.2014.10.014.
American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders Dsm-IV-Tr (Text Revision) (4th edn. ed.). Arlington: American Psychiatric Press Inc.
Anagnostou, E., & Taylor, M. J. (2011). Review of neuroimaging in autism spectrum disorders: What have we learned and where we go from here. Molecular Autism, 2(1), 4. doi:10.1186/2040-2392-2-4.
Aziz-Zadeh, L., Cattaneo, L., Rochat, M., & Rizzolatti, G. (2005). Covert speech arrest induced by rTMS over both motor and nonmotor left hemisphere frontal sites. Journal of Cognitive Neuroscience, 17(6), 928–938. doi:10.1162/0898929054021157.
Aziz-Zadeh, L., Iacoboni, M., Zaidel, E., Wilson, S., & Mazziotta, J. (2004). Left hemisphere motor facilitation in response to manual action sounds. The European Journal of Neuroscience 0953-816X (P), 19(9), 2609–2612. doi:10.1111/j.0953-816X.2004.03348.x.
Aziz-Zadeh, L., Koski, L., Zaidel, E., Mazziotta, J., & Iacoboni, M. (2006). Lateralization of the human mirror neuron system. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 26(11), 2964–2970. doi:10.1523/JNEUROSCI.2921-05.2006.
Aziz-Zadeh, L., Maeda, F., Zaidel, E., Mazziotta, J., & Iacoboni, M. (2002). Lateralization in motor facilitation during action observation: A TMS study. Experimental Brain Research. Experimentelle Hirnforschung. Experimentation cerebrale, 144(1), 127–131. doi:10.1007/s00221-002-1037-5.
Bakroon, A., & Lakshminarayanan, V. (2016). Visual function in autism spectrum disorders: A critical review. Clinical & Experimental Optometry: Journal of the Australian Optometrical Association, 99(4), 297–308. doi:10.1111/cxo.12383.
Baron-Cohen, S., Hoekstra, R. A., Knickmeyer, R., & Wheelwright, S. (2006). The autism-spectrum quotient (AQ)—Adolescent version. Journal of Autism and Developmental Disorders, 36(3), 343–350. doi:10.1007/s10803-006-0073-6.
Baron-Cohen, S., Richler, J., Bisarya, D., Gurunathan, N., & Wheelwright, S. (2003). The systemizing quotient: An investigation of adults with Asperger syndrome or high-functioning autism, and normal sex differences. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 358(1430), 361–374. doi:10.1098/rstb.2002.1206.
Baron-Cohen, S., & Wheelwright, S. (2004). The empathy quotient: An investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. Journal of Autism and Developmental Disorders, 34(2), 163–175.
Beck, A. T., Ward, C. H., Mendelson, M., Mock, J., & Erbaugh, J. (1961). An inventory for measuring depression. Archives of General Psychiatry, 4, 561–571.
Behrens, T. E., Berg, H. J., Jbabdi, S., Rushworth, M. F., & Woolrich, M. W. (2007). Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage, 34(1), 144–155. doi:10.1016/j.neuroimage.2006.09.018.
Ben Bashat, D. (2011). Abnormal developmental trajectories of white matter in autism—The contribution of MRI. In V. Eapen (Ed.), Autism—A Neurodevelopmental journey from genes to behaviour: Rijeka: InTech.
Buck, T. R., Viskochil, J., Farley, M., Coon, H., McMahon, W. M., Morgan, J., et al. (2014). Psychiatric comorbidity and medication use in adults with autism spectrum disorder. Journal of Autism and Developmental Disorders, 44(12), 3063–3071. doi:10.1007/s10803-014-2170-2.
Cheng, Y., Chou, K. H., Decety, J., Chen, I. Y., Hung, D., Tzeng, O. J., et al. (2009). Sex differences in the neuroanatomy of human mirror-neuron system: A voxel-based morphometric investigation. Neuroscience, 158(2), 713–720. doi:10.1016/j.neuroscience.2008.10.026.
Chien, H. Y., Gau, S. S., Hsu, Y. C., Chen, Y. J., Lo, Y. C., Shih, Y. C., et al. (2015). Altered cortical thickness and tract integrity of the mirror neuron system and associated social communication in autism spectrum disorder. Autism Research, 8(6), 694–708. doi:10.1002/aur.1484.
Christensen, D. L., Baio, J., Braun, K. V., Bilder, D., Charles, J., Constantino, J. N., et al. (2016). Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR. Surveillance Summaries: Morbidity and Mortality Weekly Report. Surveillance Summaries/CDC, 65(3), 1–23. doi:10.15585/mmwr.ss6503a1.
Dapretto, M., Davies, M. S., Pfeifer, J. H., Scott, A. A., Sigman, M., Bookheimer, S. Y., et al. (2006). Understanding emotions in others: Mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience, 9(1), 28–30. doi:10.1038/nn1611.
De Fosse, L., Hodge, S. M., Makris, N., Kennedy, D. N., Caviness, V. S. Jr., McGrath, L., et al. (2004). Language-association cortex asymmetry in autism and specific language impairment. Annals of Neurology, 56(6), 757–766. doi:10.1002/ana.20275.
Fan, Y. T., Decety, J., Yang, C. Y., Liu, J. L., & Cheng, Y. (2010). Unbroken mirror neurons in autism spectrum disorders. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 51(9), 981–988. doi:10.1111/j.1469-7610.2010.02269.x.
Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., et al. (2002). Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355.
Goodman, W. K., Price, L. H., Rasmussen, S. A., Mazure, C., Fleischmann, R. L., Hill, C. L., et al. (1989). The Yale-Brown obsessive compulsive scale. I. Development, use, and reliability. Archives of General Psychiatry, 46(11), 1006–1011.
Hadjikhani, N., Joseph, R. M., Snyder, J., & Tager-Flusberg, H. (2006). Anatomical differences in the mirror neuron system and social cognition network in autism. Cerebral Cortex (New York, N. Y.: 1991), 16(9), 1276–1282. doi:10.1093/cercor/bhj069.
Hamilton, A. F. (2008). Emulation and mimicry for social interaction: A theoretical approach to imitation in autism. The Quarterly Journal of Experimental Psychology, 61(1), 101–115. doi:10.1080/17470210701508798.
Hamilton, A. F. (2013). Reflecting on the mirror neuron system in autism: A systematic review of current theories. Developmental Cognitive Neuroscience 3, 91–105. doi:10.1016/j.dcn.2012.09.008.
Hamilton, A. F. (2015). Cognitive underpinnings of social interaction. The Quarterly Journal of Experimental Psychology, 68(3), 417–432. doi:10.1080/17470218.2014.973424.
Hamilton, A. F., & Grafton, S. T. (2007). The motor hierarchy: From kinematics to goals and intentions. In P. Haggard;, Y. Rosetti; & M. Kawato, (Eds.), Attention and Performance xxii (pp. 1–29). Oxford: Oxford University Press.
Haxby, J. V., Hoffman, E. A., & Gobbini, M. I. (2000). The distributed human neural system for face perception. Trends in cognitive sciences, 4(6), 223–233.
Hecht, E. E., Gutman, D. A., Preuss, T. M., Sanchez, M. M., Parr, L. A., & Rilling, J. K. (2013). Process versus product in social learning: Comparative diffusion tensor imaging of neural systems for action execution-observation matching in macaques, chimpanzees, and humans. Cerebral cortex (New York, N. Y.: 1991), 23(5), 1014–1024. doi:10.1093/cercor/bhs097.
Herbert, M. R., Harris, G. J., Adrien, K. T., Ziegler, D. A., Makris, N., Kennedy, D. N., et al. (2002). Abnormal asymmetry in language association cortex in autism. Annals of Neurology, 52(5), 588–596. doi:10.1002/ana.10349.
Hirose, K., Miyata, J., Sugihara, G., Kubota, M., Sasamoto, A., Aso, T., et al. (2014). Fiber tract associated with autistic traits in healthy adults. Journal of Psychiatric Research, 59, 117–124. doi:10.1016/j.jpsychires.2014.09.001.
Iacoboni, M. (2005). Neural mechanisms of imitation. Current Opinion in Neurobiology, 15(6), 632–637. doi:10.1016/j.conb.2005.10.010.
Iacoboni, M., & Dapretto, M. (2006). The mirror neuron system and the consequences of its dysfunction. Nature reviews Neuroscience, 7(12), 942–951. doi:10.1038/nrn2024.
Iacoboni, M., Molnar-Szakacs, I., Gallese, V., Buccino, G., Mazziotta, J. C., & Rizzolatti, G. (2005). Grasping the intentions of others with one’s own mirror neuron system. PLoS Biology, 3(3), e79. doi:10.1371/journal.pbio.0030079.
Iacoboni, M., Woods, R. P., Brass, M., Bekkering, H., Mazziotta, J. C., & Rizzolatti, G. (1999). Cortical mechanisms of human imitation. Science, 286(5449), 2526–2528.
Izawa, J., Pekny, S. E., Marko, M. K., Haswell, C. C., Shadmehr, R., & Mostofsky, S. H. (2012). Motor learning relies on integrated sensory inputs in ADHD, but over-selectively on proprioception in autism spectrum conditions. Autism Research, 5(2), 124–136. doi:10.1002/aur.1222.
Lehrl, S. (2005). Mehrfachwach-Wortschatz-Intelligenztest MWT-B (5th edn). Balingen: Spitta.
Martineau, J., Andersson, F., Barthelemy, C., Cottier, J. P., & Destrieux, C. (2010). Atypical activation of the mirror neuron system during perception of hand motion in autism. Brain Research, 1320, 168–175. doi:10.1016/j.brainres.2010.01.035.
Mattis, S. (1988). Dementia rating scale: Professional manual. Odessa, FL: Psychological Assessment Resources
Nebel, M. B., Eloyan, A., Nettles, C. A., Sweeney, K. L., Ament, K., Ward, R. E., et al. (2016). Intrinsic visual-motor synchrony correlates with social deficits in autism. Biological Psychiatry, 79(8), 633–641. doi:10.1016/j.biopsych.2015.08.029.
Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113.
Ramachandran, V. S., & Oberman, L. M. (2006). Broken mirrors: A theory of autism. Scientific American, 295(5), 62–69.
Retz-Junginger, P., Retz, W., Blocher, D., Weijers, H. -G., Trott, G. -E., Wender, P. H., Rössler, M. (2002). Wender Utah Rating Scale (WURS-k) Die deutsche Kurzform zur retrospektiven Erfassung des hyperkinetischen Syndroms bei Erwachsenen. Der Nervenarzt, 73(9), 830–838.
Rizzolatti, G., Cattaneo, L., Fabbri-Destro, M., & Rozzi, S. (2014). Cortical mechanisms underlying the organization of goal-directed actions and mirror neuron-based action understanding. Physiological Reviews, 94(2), 655–706. doi:10.1152/physrev.00009.2013.
Rizzolatti, G., & Craighero, L. (2004a). The mirror-neuron system. Annual Review of Neuroscience, 27, 169–192. doi:10.1146/annurev.neuro.27.070203.144230.
Rizzolatti, G., & Craighero, L. (2004b). The mirror-neuron system. Annual Review of Neuroscience, 27, 169–192. doi:10.1146/annurev.neuro.27.070203.144230.
Rizzolatti, G., Fabbri-Destro, M., & Cattaneo, L. (2009). Mirror neurons and their clinical relevance. Nature Clinical Practice. Neurology, 5(1), 24–34. doi:10.1038/ncpneuro0990.
Rizzolatti, G., Fadiga, L., Matelli, M., Bettinardi, V., Paulesu, E., Perani, D., et al. (1996). Localization of grasp representations in humans by PET: 1. Observation versus execution. Experimental Brain Research. Experimentelle Hirnforschung. Experimentation Cerebrale, 111(2), 246–252.
Rosler, M., Retz, W., Retz-Junginger, P., Thome, J., Supprian, T., Nissen, T., et al. (2004). [Tools for the diagnosis of attention-deficit/hyperactivity disorder in adults. Self-rating behaviour questionnaire and diagnostic checklist]. Der Nervenarzt, 75(9), 888–895. doi:10.1007/s00115-003-1622-2.
Sasaki, A. T., Kochiyama, T., Sugiura, M., Tanabe, H. C., & Sadato, N. (2012). Neural networks for action representation: A functional magnetic-resonance imaging and dynamic causal modeling study. Frontiers in Human Neuroscience, 6, 236. doi:10.3389/fnhum.2012.00236.
Sato, W., Toichi, M., Uono, S., & Kochiyama, T. (2012). Impaired social brain network for processing dynamic facial expressions in autism spectrum disorders. BMC Neuroscience, 13, 99. doi:10.1186/1471-2202-13-99.
Schaer, M., Ottet, M. C., Scariati, E., Dukes, D., Franchini, M., Eliez, S., et al. (2013). Decreased frontal gyrification correlates with altered connectivity in children with autism. Frontiers in Human Neuroscience, 7, 750. doi:10.3389/fnhum.2013.00750.
Schaipp, C. (2001). Validität und diagnostische Brauchbarkeit ausgewählter indirekter und direkter Befragungsmethoden zur Diagnostik von Aggressivität, Neurotizismus bzw. psychischer Stabilität. München: Herbert Utz Verlag.
Schulz, R., Frey, B. M., Koch, P., Zimerman, M., Bonstrup, M., Feldheim, J., et al. (2015a). Cortico-cerebellar structural connectivity is related to residual motor output in chronic stroke. Cerebral cortex (New York, N. Y.: 1991). doi:10.1093/cercor/bhv251.
Schulz, R., Koch, P., Zimerman, M., Wessel, M., Bonstrup, M., Thomalla, G., et al. (2015b). Parietofrontal motor pathways and their association with motor function after stroke. Brain: A Journal of Neurology, 138(Pt 7), 1949–1960. doi:10.1093/brain/awv100.
Schunke, O., Schottle, D., Vettorazzi, E., Brandt, V., Kahl, U., Baumer, T., et al. (2015). Mirror me: Imitative responses in adults with autism. Autism: The International Journal of Research and Practice. doi:10.1177/1362361315571757.
Shih, P., Keehn, B., Oram, J. K., Leyden, K. M., Keown, C. L., & Muller, R. A. (2011). Functional differentiation of posterior superior temporal sulcus in autism: A functional connectivity magnetic resonance imaging study. Biological psychiatry, 70(3), 270–277. doi:10.1016/j.biopsych.2011.03.040.
Southgate, V., & Hamilton, A. F. (2008). Unbroken mirrors: Challenging a theory of autism. Trends in cognitive sciences, 12(6), 225–229. doi:10.1016/j.tics.2008.03.005.
Spek, A., Schatorje, T., Scholte, E., & van Berckelaer-Onnes, I. (2009). Verbal fluency in adults with high functioning autism or Asperger syndrome. Neuropsychologia, 47(3), 652–656. doi:10.1016/j.neuropsychologia.2008.11.015.
Travers, B. G., Adluru, N., Ennis, C., Tromp do, P. M., Destiche, D., Doran, S., et al. (2012). Diffusion tensor imaging in autism spectrum disorder: A review. Autism Research, 5(5), 289–313. doi:10.1002/aur.1243.
Verly, M., Verhoeven, J., Zink, I., Mantini, D., Van Oudenhove, L., Lagae, L., et al. (2014). Structural and functional underconnectivity as a negative predictor for language in autism. Human Brain Mapping, 35(8), 3602–3615. doi:10.1002/hbm.22424.
von dem Hagen, E. A., Nummenmaa, L., Yu, R., Engell, A. D., Ewbank, M. P., & Calder, A. J. (2011). Autism spectrum traits in the typical population predict structure and function in the posterior superior temporal sulcus. Cerebral cortex (New York, N. Y.: 1991), 21(3), 493–500. doi:10.1093/cercor/bhq062.
Wheelwright, S., Baron-Cohen, S., Goldenfeld, N., Delaney, J., Fine, D., Smith, R., et al. (2006). Predicting autism spectrum quotient (AQ) from the systemizing quotient-revised (SQ-R) and empathy quotient (EQ). Brain Research, 1079(1), 47–56. doi:10.1016/j.brainres.2006.01.012.
Williams, J. H., Whiten, A., Suddendorf, T., & Perrett, D. I. (2001). Imitation, mirror neurons and autism. Neuroscience and Biobehavioral Reviews, 25(4), 287–295.
Wittchen, H. U., Zaudig, M., & Fydrich, T. (1997). Strukturiertes Klinisches Interview für DSM-IV. Göttingen: Hogrefe.
Yang, J., & Hofmann, J. (2015). Action observation and imitation in autism spectrum disorders: An ALE meta-analysis of fMRI studies. Brain Imaging and Behavior. doi:10.1007/s11682-015-9456-7.
Acknowledgments
We would like to thank the patients for their participation in the study.
Funding
This study was supported by the Else Kröner-Fresenius-Stiftung (2011_A37; AM), by the Deutsche Forschungsgemeinschaft (DFG; SFB 936/A3/C5, A.M., A.K.E.) and by the European Union (EU, “socSMCs” - H2020-641321, AKE).
Author information
Authors and Affiliations
Contributions
OF conceived of the study, participated in its design and coordination, performed the measurement and parts of the statistical analysis, interpreted the data and drafted and revised the manuscript. RS participated in its design, performed the measurement and parts of the statistical analysis, interpreted the data and drafted and revised the manuscript. DS, ND and IP conceived of the study, participated in its design and coordination, performed the measurement and revised the manuscript. BC, GT, HB, CG, AE, TB participated in the design and coordination of the study, interpreted the data and revised the manuscript. AM supervised the study, conceived of the study, participated in its design and coordination, performed the measurement interpreted the data and drafted and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Odette Fründt received conference and travel funds for the MDS Congress in Berlin in 2016 by BIAL. Daniel Schöttle received honoria for speaking at symposia and attending symposia by Janssen-Cilag, Otsuka and Lundbeck. Götz Thomalla received fees as a consultant, lecture fees, or advisory board participation from Acandis, Bristol-Myers Squibb/Pfizer, Boehringer Ingelheim, Daichii Sankyo, GlaxoSmithKline, and Stryker. He received a research grant by Bayer. Christos Ganos received research support from the German Parkinson Society, and the German Research Foundation (Deutsche Forschungsgemeinschaft) and is currently funded by the VolkswagenStiftung (Freigeist-Fellowship). Besides the abovementioned funding by the European Union and the DFG (EU, “socSMCs” - H2020-641321, DFG; SFB 936/A3/C5), Ina Peiker, Nicole David and Andreas K. Engel do not have any other conflicts of interest. Tobias Bäumer received honaria for speaking at symposia form Merz Pharmaceuticals, Ipsen Pharma, Allergan and Child & Brain and served on the scientific advisory board for Merz Pharmaceuticals. Alexander Münchau served on the scientific advisory board of the Tourette Gesellschaft Deutschland (German Tourette syndrome association) and was speaker of the Lübeck Centre for Rare Diseases. He received research grants by Pharm Allergan, Ipsen, Merz Pharmaceuticals, Actelion and got honoraria for lectures from Pharm Allergan, Ipsen, Merz Pharmaceuticals, Actelion, GlaxoSmithKline, Desitin and Teva. He furthermore obtained support from non-profit foundations or societies Possehl-Stiftung, Lübeck; Dystonia Coalition (USA); Margot und Jürgen Wessel Stiftung (Lübeck), Tourette Syndrome Association (Germany); European Huntington Disease Network; N.E.MO. Charity supporting the research of paediatric movement disorders; Ärztekammer Schleswig-Holstein; Fortbildungsakademie der Deutschen Gesellschaft für Neurologie; Förderstiftung des Universitätsklinikums Schleswig-Holstein (UKSH). He furthermore received academic research support by the European Union as part of the FP 7 program (HEALTH.2011.2.2.1-3, PI, 2011–2015), the Deutsche Forschungsgemeinschaft (DFG, MU 1692/3-1, PI, 2010–2014/SFB 936, PI, 2011–2015 and 2015–2019/MU 1692/4-1, PI, 2015–2017) and the Bundesministerium für Bildung und Forschung (BMBF): DysTract consortium, PI, 2015–2018. He got royalties from the publication of the book Neurogenetics (Oxford University Press). Robert Schulz, Bastian Cheng and Hanna Braaß do not have any conflicts of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Odette Fründt and Robert Schulz have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fründt, O., Schulz, R., Schöttle, D. et al. White Matter Microstructure of the Human Mirror Neuron System is Related to Symptom Severity in Adults with Autism. J Autism Dev Disord 48, 417–429 (2018). https://doi.org/10.1007/s10803-017-3332-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-017-3332-9