Abstract
An existing randomised controlled trial was used to investigate whether multiple ultrasound scans may be associated with the autism phenotype. From 2,834 single pregnancies, 1,415 were selected at random to receive ultrasound imaging and continuous wave Doppler flow studies at five points throughout pregnancy (Intensive) and 1,419 to receive a single imaging scan at 18 weeks (Regular), with further scans only as indicated on clinical grounds. There was no significant difference in the rate of Autism Spectrum Disorder between the Regular (9/1,125, 0.8 %) and Intensive (7/1,167, 0.6 %) groups, nor a difference between groups in the level of autistic-like traits in early adulthood. There is no clear link between the frequency and timing of prenatal ultrasound scans and the autism phenotype.
Similar content being viewed by others
Introduction
Autism Spectrum Disorder (ASD) is the collective term for neurodevelopmental disorders that are characterized by qualitative impairments in social interaction and communication, and a restricted range of activities and interests. Although the biological pathways contributing to ASD remain poorly understood, current consensus is for a multifactorial etiology, incorporating a constellation of genetic risk variants that interact with environmental factors (Persico and Bourgero 2006). Rates of diagnosis of ASD have risen markedly over the past three decades from 21.2 per 10,000 (0.212 %) in 1979 (Wing and Gould 1979) to 62.5 per 10,000 (0.625 %) in 2006 (MacDermott et al. 2007). There is vigorous debate as to what proportion of the increase in prevalence is an artefact of the broadening of diagnostic boundaries (Bishop et al. 2008) and greater public awareness (Leonard et al. 2010), and what proportion of the rise is genuine, perhaps reflecting increased exposure to one or more environmental teratogens (Landrigan 2010; London and Etzel 2000).
The rise in the popularity and use of prenatal ultrasonography is one environmental factor hypothesized to be associated with the increase in ASD prevalence (Olson 2009; Rodgers 2006; Williams and Casanova 2010). Prenatal ultrasonography was introduced into obstetrical practice in the 1970s (Huang et al. 2006), and multiple ultrasound scans have become commonplace as a means of charting fetal growth and development. Ultrasound uses high-frequency sound which, when absorbed, may lead to thermal (hyperthermia) and non-thermal (cavitation and streaming) effects on localized cells with potentially adverse consequences on fetal central nervous system development (Church and Miller 2007). Williams and Casanova (2010) proposed that prenatal ultrasonography may provide an additional environmental stressor to individuals with a genetic susceptibility to ASD during a critical period of neurodevelopment, and thus further increase their risk for disorder.
A number of studies have investigated the effects of prenatal ultrasaound scans on neurodevelopment. Using Swedish population data, Kieler et al. (2001) found that non-right handedness was more common among offspring exposed to prenatal ultrasonography, suggesting that ultrasound imaging studies may exert an effect on fetal brain development. However, further investigations by this research group identified no link between prenatal ultrasonography and cognitive performance (Stålberg et al. 2009), or the prevalence of schizophrenia (Stålberg et al. 2007) among offspring.
ASD cannot be reliably diagnosed until after birth, and thus prospective investigations of prenatal life have been rare. One study retrospectively compared the frequency and timing of prenatal ultrasound scans among children later diagnosed with ASD and matched typically developing controls, reporting largely null findings (Grether et al. 2010). However, information on the type of ultrasonography the women received could not be gleaned from medical records in approximately 25 % of scans, and therefore only limited conclusions can be drawn from these data. There has been a clear need for prospective randomised controlled trials to examine whether the frequency and/or timing of prenatal ultrasound scans is associated with the ASD phenotype among offspring.
The current study provides a follow-up of a randomized-controlled trial of repeated ultrasound scans on 2,900 pregnant women and the effects on offspring development that commenced in 1989 (Newnham et al. 2004; Newnham et al. 1993). Women were allocated to one of two groups: the Intensive group, in which women received ultrasound imaging and continuous wave Doppler flow studies at five different time-points throughout pregnancy, or the Regular group, in which women received a single imaging scan in the second trimester. Both groups received further scans only as indicated on clinical grounds. At birth, the Intensive group had a small but significantly higher risk of experiencing intrauterine growth restriction (odds ratio = 1.65, 95 % confidence intervals: 1.09–2.49) (Newnham et al. 1993). However, by 1 year of age, the physical sizes of offspring from the two groups were similar, with further follow-ups finding no significant differences in standard tests of speech, language, and behavioural development to 8 years of age (Newnham et al. 2004).
The current study examined the ASD phenotype in offspring of women in the Regular and Intensive groups. The rate of clinically-diagnosed ASD in the two groups is reported. However, because the relatively small number of participants (by epidemiological standards) in the two groups enabled only large effects to be detected, we also examined autistic-like traits in the broader cohort. Population-based studies have provided support for a smooth continuum of autistic-like traits across the general population, with clinical ASD representing the extreme end of a quantitative distribution (Constantino and Todd 2003; Happé et al. 2006; Ronald et al. 2006). These findings are robust, having been observed in both children (Constantino and Todd 2003; Ronald et al. 2006) and adults (Baron-Cohen et al. 2001; Whitehouse et al. 2011), using a variety of assessment tools (Happé et al. 2006). Inherent in the continuum view of ASD is that the biological mechanisms that are associated with clinical disorder at the extremes of the distribution may also underpin variation in autistic-like traits within the general population (Plomin et al. 2009). Thus, the aim of the current study was two-fold: To determine whether the frequency and/or timing of prenatal ultrasound scans were associated with (a) a clinical diagnosis of ASD (large effects only), and (b) the level of autistic-like traits in adults without a diagnosis of ASD.
Methods
Participants
Recruitment of the Western Australian Pregnancy (Raine) cohort has previously been described in detail (Newnham et al. 1993). In brief, following local ethics board approval, 2,900 pregnant women were recruited between 16 and 18 weeks gestation from the public antenatal clinic at King Edward Memorial Hospital (Perth, Western Australia) or nearby private practices. The criteria for enrollment were gestational age between 16 and 18 weeks, English language skills sufficient to understand the implications of participation, an expectation to deliver at KEMH, and an intention to remain in Western Australia to facilitate future follow-ups of their child. The potential for introducing bias by using a tertiary referral center population was minimized by enrolling only women who booked for antenatal care before 18 weeks gestation, thus excluding those referred for complications. Participant recruitment and all follow-ups of the study families were approved by the Human Ethics Committee at KEMH and/or Princess Margaret Hospital for Children in Perth. After complete description of the study, written informed consent was obtained from the mothers prior to the commencement of the ultrasound scans, and from the offspring at the 20-year follow-up. Singleton pregnancies only were included in this study.
Enrollment and Randomization
Women were enrolled by one of three research midwives, who assisted the women to complete a questionnaire enquiring about their social and economic circumstances, lifestyle, medical history and environmental exposures. Women were then allocated to one of two groups by a sealed-envelope technique prepared in blocks of 20 with computer generated random numbers. The Regular group had ultrasound imaging scans at 18 weeks, while women allocated to the Intensive group had an ultrasound examination and Doppler flow studies at approximately 18 weeks, and then at 24, 28, 34 and 38 weeks.
Ultrasound Studies
Ultrasound examinations were completed by a qualified sonographer using one of two General Electric 3600 machines (Milwaukee, USA) with 3.5 MHz linear array and 5 MHz sector transducers. Gestational age was calculated from the date of the last menstrual period and confirmed via ultrasound biometry at 18 weeks. Where there was discrepancy between the two estimates by more than 7 days or when women were not certain of their last normal menstrual period, gestational age was calculated using fetal ultrasound biometrics at 18 weeks. After the ultrasound imaging study, each woman in the Intensive group had a Doppler flow-velocity waveform study (Medasonics SP25A: Mountain View, California) and using a D10 bi-directional continuous wave Doppler system (total power output 3 mW, spatial peak temporal average 25 mW/cm2).
ASD Diagnosis
At the 5-, 8-, 10-, 14- and 17-year follow-ups of the Raine cohort, parents were asked whether their child had ever received a diagnosis of ASD by a health professional. Diagnosis of these conditions in Western Australia mandates assessment by a clinical team comprising a Pediatrician, Psychologist and Speech-Language Pathologist under DSM-IV guidelines (American Psychiatric Association 1994). A diagnosis is only made when there is consensus amongst the team.
Autistic-like Traits
At age 19–20 years, the cohort was invited to complete the Autism Spectrum Quotient (AQ). Adults with a known diagnosis of any developmental disorder (including ASD) were not invited to complete the AQ due to ethical concerns. The AQ is a self-report questionnaire that provides a quantitative measure of autistic-like traits in the general population (Baron-Cohen et al. 2001). Individuals are provided with 50 statements and asked to indicate on a 4-point scale how well that statement applied to them (strongly agree, agree, disagree, strongly disagree). The statements are divided into five subscales: Social Skills (‘I prefer to do things with others rather than on my own’), Attention to Detail (‘I am fascinated by numbers’), and Attention Switching (‘I enjoy doing things spontaneously’), Communication (‘People often tell me that I keep going on and on about the same thing’) and Imagination (‘I like to collect information about categories of things). Items within each subscale are then summed to provide a quantitative measure of that particular AT, with higher scores denoting increased symptomatology. The AQ has been shown to have good psychometric properties and discriminate well between high-functioning individuals with ASD and those unaffected by the disorder (Baron-Cohen et al. 2001; Woodbury-Smith et al. 2005).
Covariates
A range of sociodemographic and obstetric variables were also recorded at 18-weeks pregnancy (maternal age at conception, maternal body mass index, maternal completion of secondary school, family income below the poverty threshold of $AUD24,000), 34-weeks pregnancy (maternal smoking and alcohol consumption during pregnancy) and birth (offspring sex and parity).
Statistical Analysis
The analyses first sought to compare the rate of clinically diagnosed ASD among offspring from the Regular and Intensive groups, with the difference expressed as an odds ratio with 95 % confidence intervals (95 %CI). We then investigated the association between the prenatal ultrasound groups and autistic-like traits within the broader cohort. Multivariate linear regression analyses (including sociodemographic and obstetric covariates) were conducted to investigate AQ scores as continuous variables, and multivariate logistic regression (including covariates) and Chi-square analyses investigated AQ scores as dichotomous variables, incorporating a threshold of the top 10 % of scores to denote ‘high levels’ of autistic-like traits. Thresholds were calculated based on AQ data from participants in the Regular and Intensive groups combined. Due to clinical necessity, the actual number of ultrasounds received by a minority of women differed from the original protocol. Thus, further multivariate logistic regression analyses of the dichotomous Total AQ variable were conducted to examine associations with the number of prenatal ultrasound scans received during the entire pregnancy, and then separately for each trimester.
Results
Clinical Diagnosis of ASD
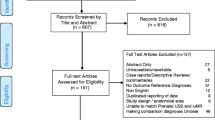
Previous analyses have reported no difference between the Regular and Intensive groups in terms of sociodemographic characteristics (Newnham et al., 1993). Postnatal ASD diagnosis data were available at any of the 5, 8, 10, 14 or 17 year follow-ups for 2,292 offspring (see Fig. 1). Participant attrition was slightly more common for offspring from the Regular group (n = 294, 20.4 %) compared to the Intensive group (n = 248, 16.8 %), χ2 = 4.67, df = 1, p = 0.03. The 542 offspring who were lost to follow-up were significantly more likely to have mothers who had not completed secondary school (lost to follow-up: 74.7 %; postnatal data available: 58.3 %; χ2 = 49.96, df = 1, p < 0.01), and who were living in a family with a household income below the poverty line (lost to follow-up: 65.3 %; postnatal data available: 39.8 %; χ2 = 115.53, df = 1, p < 0.01). Among the 2,292 participants with diagnosis data at any follow-up, 1,984 (88.3 %) contributed data at age 8 years or older, and 1,862 (82.9 %) at age 10 years or older.
Parent-report indicated that seven offspring had been diagnosed with Autism, one with Pervasive Developmental Disorder—Not Otherwise Specified (PDD-NOS) and eight with Asperger’s Syndrome, with an overall ASD prevalence of 0.7 % (16/2,292). Nine of these 16 children with ASD were in the Regular group (one female and four males with autism and one female and three males with Asperger’s Syndrome), and seven were part of the Intensive group (one male and one female with autism, one male with PDD-NOS and four males with Asperger’s Syndrome), which yielded a statistically non-significant odds ratio of 0.78 (95 % CI = 0.29, 2.1; p = 0.62).
Autistic-like Traits
Table 1 presents characteristics of the Raine sample with and without AQ data. The AQ was completed by 1,181 of the 2,292 singleton offspring (Regular group: n = 595; Intensive group = 586) with available postnatal data (see Fig. 1), with a mean age at completion of 19.64 years (SD = 0.71). There was no significant difference in the attrition rate between the Regular (n = 541, 48.1 %) and Intensive (n = 594, 50.9 %) groups, χ2 = 1.64, df = 1, p = 0.20. Offspring who did not complete the AQ were more likely to have younger mothers, overweight mothers, come from households earning below the poverty line during the mother’s pregnancy, have mothers who had not completed secondary-school and who smoked cigarettes and drank alcohol during pregnancy. The only difference between the Regular and Intensive groups was that the former group were more likely to have mothers who had not completed secondary school.
Multivariate linear regression analyses revealed no statistically significant association between the prenatal ultrasound groups and scores on the AQ (Table 2). Frequency analysis found that scores greater than or equal to 22, 5. 5, 9, 7 and 5, corresponded to the upper decile of the distribution of scores on the Total AQ (12.2 % of participants), Social Skills subscale (8 %), Communication subscale (9.4 %), Attention to Detail subscale (6.6 %), Attention Switching subscale (9.7 %) and Imagination subscale (10.7 %), respectively. Multivariate logistic regression found no significant association between the prenatal ultrasound group and the likelihood of scoring above these thresholds (Table 3).
Women received additional prenatal ultrasound scans if clinically indicated, and thus the actual number of scans administered differed from the original protocol. Figure 2 shows the proportion of offspring with a high Total AQ (score ≥ 22) according to the number of prenatal ultrasounds administered during each trimester. Multivariate logistic regression analyses found that the likelihood of an individual scoring above the threshold was not associated with the actual number or ultrasounds administered in the first-, β = −0.02, t(1,140) = 0.70 p = 0.49, second-, β = 0.00, t(1,140) = 0.07 p = 0.94, or third-, β = −0.03, t(1,140) = 1.02 p = 0.31, trimester, or the total number of ultrasound imaging scans received during the entire pregnancy, β = −0.03, t(1,140) = 0.88 p = 0.38.
Effect of Sample Attrition
Sample attrition meant that participants who completed the AQ were not representative of the original cohort in terms of sociodemographic or obstetric factors. Chi-square analyses examined whether the proportion of individuals in the current study above the Total AQ threshold for high scores (≥22) differed according to these factors. There was no statistically significant difference for any sociodemographic or obstetric variable (all p values >0.1).
Discussion
Concerns have been raised that prenatal ultrasonography may be associated with the ASD phenotype among offspring. To investigate this hypothesis, the current study utilized a randomized control design, in which half of a large sample of pregnant women had received a single ultrasound imaging scan (Regular group), and the other half received five imaging scans and continuous wave Doppler flow studies (Intensive group), with additional ultrasounds administered as clinically directed (for both groups). Of the 16 children with a clinical diagnosis of ASD, nine were from the Regular group and seven from the Intensive group; a non-significant difference. However, because the sample size only enabled the detection of large effects on clinical ASD, a measure of autistic-like traits (AQ) was also obtained from the broader offspring sample in early adulthood. Again, there was no evidence that prenatal ultrasonography was associated with increased levels of autistic-like traits, both when this variable was expressed continuously, and when dichotomized to identify those individuals the upper decile of the distribution of AQ scores. Collectively, these findings indicate that there is no clear link between prenatal ultrasonography and the ASD phenotype among offspring.
The current study utilized the gold-standard methodology of a randomized-controlled design to investigate the short- and long-term effects of prenatal ultrasonography. However, there were elements of the study design that require further discussion. Ascertainment of ASD cases within the Raine cohort was based upon parent-report of a clinician-based diagnosis (by a Pediatrician, Psychologist and Speech Pathologist) according to DSM-IV guidelines at any of the 5-, 8-, 10-, 14- or 17-year follow-ups. While it is commonplace in ASD research to confirm clinical diagnoses using ASD-specific behavioral observation and/or parent interview assessments, this was not possible within the Raine cohort. However, it is important to note that a previous investigation of developmental data obtained prior to 5 years of age (Whitehouse et al. 2011), found that each ASD case in the current study demonstrated behaviours consistent with ASD (e.g., poor eye contact, delayed language, absence of pretend play). Moreover, the overall rate of ASD within the current sample was 0.7 %, which is highly similar to the most recent population-based prevalence estimates in Australia (0.625 %) (MacDermott et al. 2007), and suggests that our reliance on clinician diagnosis did not lead to an undercount of cases.
A second point of discussion concerns the sample attrition over time, which is a common issue for longitudinal-cohort studies (Wolke et al. 2009). It is possible that participants in the current study may have been misclassified as unaffected if they received a clinical diagnosis of ASD after they were lost to follow up. However, given that ASD is typically diagnosed in early to mid-childhood (Mandell et al. 2002), we suggest that there would be very few cases in the current study, if any, where this scenario is applicable. Epidemiological studies have found that only 10 % of individuals with ASD in Australia are first diagnosed beyond 10-years of age (Williams et al. 2005), and 17.1 % of the current sample did not provide any diagnostic data at 10-years of age or older. Given these figures, we would expect (less than) one participant to have been misclassified as not having ASD.Footnote 1 Even if this misclassified participant was part of the Intensive group (i.e., Regular group = 9 ASD cases vs. Intensive group = 8 ASD cases), the odds ratio would not significantly differ from one (odds ratio: 0.89; 95 %CI: 0.34, 2.32). However, while the randomized controlled design provides an excellent opportunity to examine whether there are large effects of prenatal ultrasonography on clinical ASD, the sample sizes are too small to examine more subtle effects. Studies of total population samples with an associated increase in statistical power will provide an important addition to this area of research.
Sample attrition was also an issue for the investigation of autistic-like traits in the broader sample, where just over half of the singleton sample with available postnatal data completed the AQ in early adulthood (1,181/2,292 = 51.5 %). Whilst there may be some concern that the attrition may have reduced the representativeness of the Regular or Intensive groups, we suggest that this is not the case for four reasons. First, there was no significant difference in the rate of sample attrition between the Regular and Intensive groups. Second, while offspring were less likely to participate if they came from socially disadvantaged backgrounds (Table 1), the proportion of offspring exceeding the high AQ threshold did not vary according to these factors in the current sample. Third, the AQ scores and ranges are similar to those that have been reported in other ‘general population’ samples in the US (Hurst et al. 2007), UK (Baron-Cohen et al. 2001), and Australia (Rosbrook and Whittingham 2007). Finally, the original Raine cohort was recruited from a tertiary maternity hospital, and is known to have over-represented socially disadvantaged women (Whitehouse et al. 2010). The pattern of attrition may have actually increased the extent to which the findings can be generalized to the general community.
A third methodological issue was our inability to determine each participant’s familial risk for ASD. Williams and Casanova (2010) hypothesized that prenatal ultrasonography may provide an additional environmental stressor to individuals with a genetic susceptibility to ASD during a critical period of neurodevelopment, thereby further increasing their risk for disorder. Data on whether a parent, sibling or extended family member had been diagnosed with ASD were not collected at each follow-up of the Raine cohort, and thus we were unable to quantify each participant’s genetic liability for ASD. Given that the original sample was an accurate reflection of the patient community attending a tertiary maternity hospital (i.e., 90 % of eligible women agreed to participate), and that the randomization schedule produced two evenly matched groups in terms of sociodemographic variables (Table 1), there is no reason to believe that the Regular and Intensive ultrasound groups would differ in the proportion of individuals with a family history of ASD.
In the current randomized-controlled trial, the frequent use of prenatal ultraonography led to a slight reduction in offspring birthweight (Newnham et al. 1993), but no increase in the incidence of ASD or subthreshold autistic symptomatology. The output of the Doppler systems utilized in this study was 25 mW/cm2, which is well below the 100 mW/cm2 threshold at which studies have identified adverse biological effects on mammalian tissue (Barnett et al. 2006; Miller et al. 1998). However, technological advancements over the past two decades have led to considerable improvements in ultrasonographic capabilities, with corresponding increases in acoustic output (Barnett and Maulik 2001). The current study was not designed to investigate the use of these instruments, and there is a clear need for research into this area. Furthermore, all women in the current study received at least one prenatal ultrasound scan, and therefore the question of whether the administration of any prenatal ultrasound scans increases the risk for ASD remains to be investigated. What we can conclude from this study is that the clinical use of low output ultrasongraphy during the second and third trimesters, which represents the vast majority of prenatal ultrasound scans during pregnancy, has no obvious relation to the ASD phenotype among offspring. Future studies that explore the ASD risk associated with prenatal ultrasound scans within total-population samples are an essential next research step to build on these preliminary findings.
Notes
i.e., (A × B) × (C × D) = 0.30 participants.
A is the number of live-born singleton offspring (n = 2,821); B is the percentage of the current sample who did not provide any diagnostic data at 10 years of age or older (0.171); C is the percentage of individuals with ASD in Australia who are diagnosed beyond 10 years of age (0.10); D = the prevalence of ASD in Australia (0.00625).
References
American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: American Psychiatric Press.
Barnett, S. B., & Maulik, D. (2001). Guidelines and recommendations for safe use of Doppler ultrasound in perinatal applications. Journal of Maternal-Foetal and Neonatal Medicine, 10(2), 75–84.
Barnett, S. B., Ter Haar, G. R., Ziskin, M. C., Rott, H.-D., Duck, F. A., & Maeda, K. (2006). International recommendations and guidelines for the safe use of diagnostic ultrasound in medicine. Ultrasound in Medicine and Biology, 26(3), 355–366.
Baron-Cohen, S., Wheelwright, S., Skinner, R., Martin, J., & Clubley, E. (2001). The autism-spectrum quotient (AQ): Evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders, 31(1), 5–17.
Bishop, D. V. M., Whitehouse, A. J. O., Watt, H. J., & Line, E. A. (2008). Autism and diagnostic substitution: evidence from a study of adults with a history of developmental language disorder. Developmental Medicine and Child Neurology, 50(5), 341–345.
Church, C. C., & Miller, M. W. (2007). Quantification of risk from foetal exposure to diagnostic ultrasound. Progress in Biophysics and Molecular Biology, 93(1–3), 331–353.
Constantino, J. N., & Todd, R. D. (2003). Autistic traits in the general population: A twin study. Archives of General Psychiatry, 60(5), 524–530.
Grether, J., Li, S., Yoshida, C., & Croen, L. (2010). Antenatal ultrasound and risk of autism spectrum disorders. Journal of Autism and Developmental Disorders, 40(2), 238–245.
Happé, F., Ronald, A., & Plomin, R. (2006). Time to give up on a single explanation for autism. Nature Neuroscience, 9(10), 1218–1220.
Huang, H., Zhang, J. Y., Wakana, S., Zhang, W. H., Ren, T. B., Richards, L. J., et al. (2006). White and gray matter development in human foetal, newborn and paediatric brains. Neuroimage, 33(1), 27–38.
Hurst, R. M., Mitchell, J. T., Kimbrel, N. A., Kwapil, T. K., & Nelson-Gray, R. O. (2007). Examination of the reliability and factor structure of the autism spectrum quotient (AQ) in a non-clinical sample. Personality and Individual Differences, 43(7), 1938–1949.
Kieler, H., Cnattingius, S., Haglund, B., Palmgren, J., & Axelsson, O. (2001). Sinistrality-a side-effect of prenatal sonography: A comparative study of young men. Epidemiology, 12(6), 618–623.
Landrigan, P. J. (2010). What causes autism? Exploring the environmental contribution. Current Opinion in Pediatrics, 22(2), 219–225. 210.1097/MOP.1090b1013e328336eb328339a.
Leonard, H., Dixon, G., Whitehouse, A. J. O., Bourke, J., Aiberti, K., Nassar, N., et al. (2010). Unpacking the complex nature of the autism epidemic. Research in Autism Spectrum Disorders, 4(4), 548–554.
London, E., & Etzel, R. A. (2000). The environment as an etiologic factor in autism: A new direction for research. Environmental Health Perspectives, 108, 401–404.
MacDermott, S., Williams, K., Ridley, G., Glasson, E., & Wray, J. (2007). The prevalence of autism in Australia: Can it be established from existing data?. Sydney: Autism Advisory Board on Autism Spectrum Disorders.
Mandell, D. S., Listerud, J., Levy, S. E., & Pinto-Martin, J. A. (2002). Race differences in the age at diagnosis among medicaid-eligible children with autism. Journal of the American Academy of Child and Adolescent Psychiatry, 41(12), 1447–1453.
Miller, M. W., Brayman, A. A., & Abramowicz, J. S. (1998). Obstetric ultrasonography: A biophysical consideration of patient safety: The “rules” have changed. American Journal of Obstetrics and Gynecology, 179(1), 241–254.
Newnham, J. P., Doherty, D. A., Kendall, G. E., Zubrick, S. R., Landau, L. L., & Stanley, F. J. (2004). Effects of repeated prenatal ultrasound examinations on childhood outcome up to 8 years of age: follow-up of a randomised controlled trial. The Lancet, 364(9450), 2038–2044.
Newnham, J. P., Evans, S. F., Michael, C. A., Stanley, F. J., & Landau, L. I. (1993). Effects of frequent ultrasound during pregnancy: A randomized controlled trial. Lancet, 342(8876), 887–891.
Olson, C. D. (2009). Does prenatal ultrasound increase risk of autism? Journal of the American Osteopathic Association, 109(2), 71–72.
Persico, A. M., & Bourgeron, T. (2006). Searching for ways out of the autism maze: Genetic, epigenetic and environmental clues. Trends in Neurosciences, 29(7), 349–358.
Plomin, R., Haworth, C. M. A., & Davis, O. S. P. (2009). Common disorders are quantitative traits. Nature Reviews Genetics, 10(12), 872–878.
Rodgers, C. (2006). Questions about prenatal ultrasound and the alarming increase in autism. Midwifery Today, 90, 16–19.
Ronald, A., Happé, F., Bolton, P., Butcher, L. M., Price, T. S., Wheelwright, S., et al. (2006). Genetic heterogeneity between the three components of the autism spectrum: A twin study. Journal of American Academy of Child and Adolescent Psychiatry, 45(6), 691–699.
Rosbrook, A., & Whittingham, K. (2007). Autistic traits in the general population: What mediates the link with depressive and anxious symptomatology? Research in Autism Spectrum Disorders, 4(3), 415–424.
Stålberg, K., Axelsson, O., Haglund, B., Hultman, C. M., Lambe, M., & Kieler, H. (2009). Prenatal ultrasound exposure and children’s school performance at age 15–16: Follow-up of a randomized controlled trial. Ultrasound in Obstetrics and Gynecology, 34(3), 297–303.
Stålberg, K., Haglund, B., Axelsson, O., Cnattingius, S., Hultman, C. M., & Kieler, H. (2007). Prenatal ultrasound scanning and the risk of schizophrenia and other psychoses. Epidemiology, 18(5), 577–582.
Whitehouse, A. J. O., Hickey, M., & Ronald, A. (2011a). Are autistic traits in the general population stable across development? PLoS ONE, 6(8), e23029.
Whitehouse, A. J. O., Hickey, M., Stanley, F. J., Newnham, J. P., & Pennell, C. E. (2011b). A preliminary study of foetal head circumference growth in autism spectrum disorder. Journal of Autism and Developmental Disorders, 41, 122–129.
Whitehouse, A. J. O., Robinson, M., Zubrick, S. R., Ang, Q. W., Stanley, F. J., & Pennell, C. E. (2010). Maternal life events during pregnancy and offspring language ability in middle childhood: The Western Australian Pregnancy Cohort Study. Early Human Development, 86(8), 487–492.
Williams, E. L., & Casanova, M. F. (2010). Potential teratogenic effects of ultrasound on corticogenesis: Implications for autism. Medical Hypotheses, 75(1), 53–58.
Williams, K., Glasson, E. J., Wray, J., Tuck, M., Helmer, M., & Bower, C. I. (2005). Incidence of autism spectrum disorders in children in two Australian states. Medical Journal of Australia, 182, 108–111.
Wing, L., & Gould, J. (1979). Severe impairments of social interaction and associated abnormalities in children: Epidemiology and classification. Journal of Autism and Developmental Disorders, 9(1), 11–29.
Wolke, D., Waylen, A., Samara, M., Steer, C., Goodman, R., Ford, T., et al. (2009). Selective drop-out in longitudinal studies and non-biased prediction of behaviour disorders. The British Journal of Psychiatry, 195(3), 249–256.
Woodbury-Smith, M. R. (2005). Screening adults for Asperger syndrome using the AQ: A preliminary study of its diagnostic validity in clinical practice. Journal of Autism and Developmental Disorders, 35(3), 331.
Acknowledgments
The authors would like to acknowledge the National Health and Medical Research Council (NHMRC) for their long term contribution to funding the study over the last 20 years. Core Management of the Raine study has been funded by the University of Western Australia (UWA), Curtin University, the UWA Faculty of Medicine, Dentistry and Health Sciences, the Raine Medical Research Foundation, the Telethon Institute for Child Health Research, and the Women’s and Infants Research Foundation. Funding from Australian Rotary Health was used for the steroid analysis. AJOW (#1004065) is funded by a Career Development Fellowship from the NHMRC (#1004065). This study was partly funded by NHMRC Project Grant #1003424. These funders had no further role in study design, analysis, data interpretation or manuscript writing and submission. The authors are extremely grateful to all of the families who took part in this study and the whole Raine Study team, which includes data collectors, cohort managers, data managers, clerical staff, scientists and volunteers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stoch, Y.K., Williams, C.J., Granich, J. et al. Are Prenatal Ultrasound Scans Associated with the Autism Phenotype? Follow-up of a Randomised Controlled Trial. J Autism Dev Disord 42, 2693–2701 (2012). https://doi.org/10.1007/s10803-012-1526-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-012-1526-8