Abstract
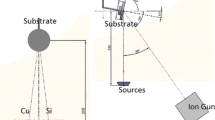
For the first time, Si–Mo–O helices have been produced by the ion-assisted glancing angle electron beam co-evaporation of molybdenum oxide and silicon. Since the electron beam evaporation process forms metastable particles through the dissociation of the source material, a film that contains compounds of different combinations of molybdenum, silicon, and oxygen atoms is produced. This complex structure’s lithiation mechanism is different from that of the traditional electrodes in lithium-ion batteries. In the paper, the nanostructured Si–Mo–O anode was cycled in different potential windows (0.2–1.2 V, 0.2–3.0 V, 5 mV–3.0 V vs. lithium) at different rates. The anode remained cycling even at 0.7 mA cm−2, which makes it practical for micro- and solid-state battery applications. This research reveals that by adjusting the cutoff voltages, different particles could be activated in the anode structure to react with lithium, resulting in different performances. The electrode delivers higher capacity when cycled between 5 mV and 3.0 V windows and keeps cycling for 200 cycles under the load of 5 µA cm−2. This performance is believed to be related to the structural, morphological, and the compositional properties of the coating.
Graphical abstract







Similar content being viewed by others
References
Wang Y, Liu B, Li Q, Cartmell S, Ferrara S, Deng ZD, Xiao J (2015) Lithium and lithium ion batteries for applications in microelectronic devices: a review. J Power Sources 286:330–345
Jin Y, Zhu B, Lu Z, Liu N, Zhu J (2017) Challenges and recent progress in the development of Si anodes for lithium-ion battery. Adv Energy Mater 7(23):1700715
Son Y, Sim S, Ma H, Choi M, Son Y, Park N, Cho J, Park M (2018) Exploring critical factors affecting strain distribution in 1D silicon-based nanostructures for lithium-ıon battery anodes. Adv Mater 30(15):1705430
Shi F, Song Z, Ross PN, Somorjai GA, Ritchie RO, Komvopoulos K (2016) Failure mechanisms of single-crystal silicon electrodes in lithium-ion batteries. Nat Commun 7:11886
Zhou G, Li H, Sun H, Yu D, Wang Y, Huang X, Chen L, Zhang Z (1999) Controlled Li doping of Si nanowires by electrochemical insertion method. Appl Phys Lett 75(16):2447–2449
Li H, Huang X, Chen L, Zhou G, Zhang Z, Yu D, Mo YJ, Pei N (2000) The crystal structural evolution of nano-Si anode caused by lithium insertion and extraction at room temperature. Solid State Ion 135(1–4):181–191
Peng K, Jie J, Zhang W, Lee S-T (2008) Silicon nanowires for rechargeable lithium-ion battery anodes. Appl Phys Lett 93(3):033105
Su X, Wu Q, Li J, Xiao X, Lott A, Lu W, Sheldon W, Wu J (2014) Adv Silicon-based nanomaterials for lithium-ion batteries: a review. Energy Mater 4(1300882):1–23
Teki R, Datta MK, Krishnan R, Parker TC, Lu TM, Kumta PN, Koratkar N (2009) Nanostructured silicon anodes for lithium ion rechargeable batteries. Small 5(20):2236–2242
Chan CK, Peng H, Liu G, McIlwrath K, Zhang XF, Huggins RA, Cui Y (2011) High-performance lithium battery anodes using silicon nanowires. In: Dusatre V (ed) Materials for sustainable energy: a collection of peer-reviewed research and review articles from nature publishing group. World Scientific Publishing Co, Singapore, pp 187–191
Holmes JD, Johnston KP, Doty RC, Korgel BA (2000) Control of thickness and orientation of solution-grown silicon nanowires. Science 287(5457):1471–1473
Sökmen Ü, Stranz A, Fündling S, Merzsch S, Neumann R, Wehmann H-H, Peiner E, Waag A (2010) Shallow and deep dry etching of silicon using ICP cryogenic reactive ion etching process. Microsyst Technol 16(5):863–870
Choi JW, McDonough J, Jeong S, Yoo JS, Chan CK, Cui Y (2010) Stepwise nanopore evolution in one-dimensional nanostructures. Nano Lett 10(4):1409–1413
Kolb F, Hofmeister H, Scholz R, Zacharias M, Gösele U, Ma D, Lee S-T (2004) Analysis of silicon nanowires grown by combining SiO evaporation with the VLS mechanism. J Electrochem Soc 151(7):G472–G475
Morales AM, Lieber CM (1998) A laser ablation method for the synthesis of crystalline semiconductor nanowires. Science 279(5348):208–211
Au M, He Y, Zhao Y, Ghassemi H, Yassar RS, Garcia-Diaz B, Adams T (2011) Silicon and silicon–copper composite nanorods for anodes of Li-ion rechargeable batteries. J Power Sources 196(22):9640–9647
Fleischauer M, Topple J, Dahn J (2005) Combinatorial investigations of Si-M (M = Cr + Ni, Fe, Mn) thin film negative electrode materials. Electrochem Solid-State Lett 8(2):A137–A140
Anani A, Huggins R (1992) Multinary alloy electrodes for solid state batteries I. A phase diagram approach for the selection and storage properties determination of candidate electrode materials. J Power Sources 38(3):351–362
Netz A, Huggins RA, Weppner W (2003) The formation and properties of amorphous silicon as negative electrode reactant in lithium systems. J Power Sources 119:95–100
Kwon Y, Kim H, Doo SG, Cho J (2007) Sn0.9 Si0.1/carbon core − shell nanoparticles for high-density lithium storage materials. Chem Mater 19(5):982–986
Courtel FM, Duguay D, Abu-Lebdeh Y, Davidson IJ (2012) Investigation of CrSi2 and MoSi2 as anode materials for lithium-ion batteries. J Power Sources 202:269–275
Tysyachny V, Shembel E, Apostolova R, Nagirny V, Kylyvnyk K, Eskova N (2004) Studies of the lithium ion transport properties in electrolytic molybdenum oxides. Solid State Ion 169(1–4):135–137
Palanisamy K, Kim Y, Kim H, Kim JM, Yoon W-S (2015) Self-assembled porous MoO2/graphene microspheres towards high performance anodes for lithium ion batteries. J Power Sources 275:351–361
Meduri P, Clark E, Kim JH, Dayalan E, Sumanasekera GU, Sunkara MK (2012) MoO3–x nanowire arrays as stable and high-capacity anodes for lithium ion batteries. Nano Lett 12(4):1784–1788
Liu Y, Zhang H, Ouyang P, Li Z (2013) One-pot hydrothermal synthesized MoO2 with high reversible capacity for anode application in lithium ion battery. Electrochim Acta 102:429–435
Ko YN, Park SB, Jung KY, Kang YC (2013) One-pot facile synthesis of ant-cave-structured metal oxide–carbon microballs by continuous process for use as anode materials in Li-ion batteries. Nano Lett 13(11):5462–5466
Hwang C-M, Lim C-H, Yang J-H, Park J-W (2009) Electrochemical properties of negative SiMox electrodes deposited on a roughened substrate for rechargeable lithium batteries. J Power Sources 194(2):1061–1067
Polat BD, Eryilmaz OL, Erck R, Keleş O, Erdemir A, Amine K (2014) Structured SiCu thin films in LiB as anodes. Thin Solid Films 572:134–141
Yao Z, Stiglich J, Sudarshan T (1999) Molybdenum silicide based materials and their properties. J Mater Eng Perform 8(3):291–304
Sidorov T (1967) Vibration spectra of three-component silicate glasses and the role of chemical elements in the structure of glass. J Appl Spectrosc 7(3):258–261
Atuchin V, Gavrilova T, Kostrovsky V, Pokrovsky L, Troitskaia I (2008) Morphology and structure of hexagonal MoO3 nanorods. Inorg Mater 44(6):622
Wang J, Zhao H, He J, Wang C, Wang J (2011) Nano-sized SiOx/C composite anode for lithium ion batteries. J Power Sources 196(10):4811–4815
Sun Y, Liu N, Cui Y (2016) Promises and challenges of nanomaterials for lithium-based rechargeable batteries. Nat Energy 1(7):16071
Sun Y, Hu X, Luo W, Huang Y (2012) Ultrafine MoO2 nanoparticles embedded in a carbon matrix as a high-capacity and long-life anode for lithium-ion batteries. J Mater Chem 22(2):425–431
Leroux F, Nazar LF (2000) Uptake of lithium by layered molybdenum oxide and its tin exchanged derivatives: high volumetric capacity materials. Solid State Ion 133(1–2):37–50
Huang X, Gan X, Huang Q, Yang J (2018) Electrochemical performance of thermally-grown SiO2 as diffusion barrier layer for integrated lithium-ion batteries. Front Energy 12(2):225–232
Liang Y, Yang S, Yi Z, Lei X, Sun J, Zhou Y (2005) Low temperature synthesis of a stable MoO2 as suitable anode materials for lithium batteries. Mater Sci Eng, B 121(1–2):152–155
Sen UK, Mitra S (2014) Synthesis of molybdenum oxides and their electrochemical properties against Li. Energy Procedia 54:740–747
Lee SH, Kim YH, Deshpande R, Parilla PA, Whitney E, Gillaspie DT, Jones KM, Mahan AH, Zhang S, Dillon AC (2008) Reversible lithium-ion insertion in molybdenum oxide nanoparticles. Adv Mater 20(19):3627–3632
Liu X, Liu Y, Yan X, Lan J-L, Yua Y, Yang X (2019) Ultrafine MoO3 nanoparticles embedded in porous carbon nanofibers as anodes for high-performance lithium-ion batteries. Mater Chem Front 3(120):120–126
Fu H, Xu Z, Wang T, Li K, Shen X, Li J, Huang J (2018) Rate behavior of MoO2/graphene oxide lithium-ıon battery anodes from electrochemical contributions. J Electrochem Soc 165(3):A439–A447
Acknowledgement
The author thanks Prof. Dr. Özgül Keleş, Dr. Levent Eryilmaz, and Dr. Robert Erck for their contributions to the study. Also to be thanked are Prof. Dr. Mehmet Ali Gülgüt, Prof. Dr. Gültekin Göller, Meltem Sezen, and Hüseyin Sezer for their help in material characterization. K.A. gratefully acknowledge support from the US Department of Energy (DOE), Office of Energy Efficiency and Renewable Energy, Vehicle Technologies Office. Argonne National Laboratory is operated for DOE Office of Science by UChicago Argonne, LLC, under contract no. DE-AC02-06CH11357.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karahan, B.D., Amine, K. Engineering self-standing Si–Mo–O based nanostructure arrays as anodes for new era lithium-ion batteries. J Appl Electrochem 49, 671–680 (2019). https://doi.org/10.1007/s10800-019-01319-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-019-01319-w