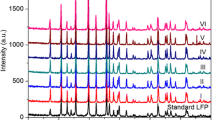
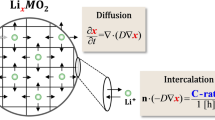
Abstract
The galvanostatic performance of a pristine lithium iron phosphate (LFP) electrode is investigated. Based on the poor intrinsic electronic conductivity features of LFP, an empirical variable resistance approach is proposed for the single particle model (SPM). The increasing resistance behavior observed at the end of discharge process of LFP batteries can be justified by the increased ohmic resistance, a resistive-reactant feature of LFP as the positive electrode active materials. The model is validated for two different laboratory made Li/LFP coin cells: a high-energy and a high-power configuration. Comparisons between the experimental results and the model predictions reveal that a variable resistance is successful to tackle the increasing overpotential.
Graphical Abstract
Schematic of the coated LFP active material particles in (a) beginning of discharge with well-connected particles, (b) end of discharge with poor-connected particles










Similar content being viewed by others
Abbreviations
- \(c_{{s,k}}^{{\max }}\) :
-
Maximum concentration of Li+ in the particle of positive electrode (mol m−3)
- \({D_{s,p}}\) :
-
Li+ diffusion coefficient in the particle of positive electrode (m2 s−1)
- \(F\) :
-
Faraday’s constant (C mol−1)
- \(I\) :
-
Applied current density, (A m−2)
- \({K_k}\) :
-
Reaction rate constant of electrode k (k = p,n), (m2.5 mol−0.5 s−1)
- P:
-
Unknown parameter vector
- \(R\) :
-
Universal gas constant (J mol−1 K−1)
- \({R_p}\) :
-
Radius of the particles of positive electrode (m)
- \({S_k}\) :
-
Total electroactive area of electrode k (k = p,n) (m2)
- \(SO{C_p}\) :
-
State of charge of positive electrode
- \(SO{C_{p,ini}}\) :
-
Initial state of charge of positive electrode
- \(t\) :
-
Time (s)
- \(T\) :
-
Absolute temperature (K)
- \({U_p}\) :
-
Open-circuit potential of positive electrode (V)
- \({V_p}\) :
-
Total volume of positive electrode (m3)
- \({{\text{V}}_{cell}}\) :
-
Model’s estimation of the cell potential (V)
- \({\varepsilon _p}\) :
-
Volume fraction of active material
- \({\delta _p}\) :
-
Dimensionless flux of lithium ion at positive electrode
- \({\lambda _k}\) :
-
The kth eigenvalue
- \(ini\) :
-
Initial state
- \(p\) :
-
Positive electrode
- \(n\) :
-
Negative electrode
- \(s\) :
-
Solid phase
References
Ravet N, Goodenough JB, Besner S, Simoneau M, Hovington P, Armand M (1999) In 96th Meeting of the Electrochemical Society, Vol. 99–2, Abstract, # 127, Hawai
Ravet N, Chouinard Y, Magnan JF, Besner S, Gauthier M, Armand M (2001) Electroactivity of natural and synthetic triphylite. J Power Sources 97:503–507
Yamada A, Chung SC, Hinokuma K (2001) Optimized LiFePO4 for lithium battery cathodes., J Electrochem Soc 148(3):A224-A229
Delacourt C, Poizot P, Levasseur S, Masquelier C (2006) Size effects on carbon-free LiFePO4 powders the key to superior energy density. Electrochem. Solid-State Lett 9:A352–A355
Islam MS, Driscoll DJ, Fisher CA, Slater PR (2005) Atomic-scale investigation of defects, dopants, and lithium transport in the LiFePO4 olivine-type battery material. Chem Mater 17(20):5085–5092
Morgan D, Van der Ven A, Ceder G (2004) Li conductivity in Li x MPO 4 (M = Mn, Fe, Co, Ni) olivine materials. Electrochem Solid-State Lett 7(2):A30–A32
Chen G, Song X, Richardson TJ (2006) Electron microscopy study of the LiFePO4 to FePO4 phase transition. Electrochem Solid-State Lett 9(6):A295–A298
Munakata H, Takemura B, Saito T, Kanamura K (2012) Evaluation of real performance of LiFePO4 by using single particle technique. J Power Sources 217:444–448
Huang H, Yin SC, Nazar LF (2001) Approaching theoretical capacity of LiFePO4 at room temperature at high rates. Electrochem Solid-State Lett 4(10):A170–A172
Laffont L, Delacourt C, Gibot P, Wu MY, Kooyman P, Masquelier C, Tarascon JM (2006) Study of the LiFePO4/FePO4 two-phase system by high-resolution electron energy loss spectroscopy. Chem Mater 18(23):5520–5529
Laffont NN, Nikolowski K, Baehtz C, Bramnik KG, Ehrenberg H (2007) Phase transitions occurring upon lithium insertion-extraction of LiCoPO4. Chem Mater 19(4):908–915
Brunetti G, Robert D, Bayle-Guillemaud P, Rouviere JL, Rauch EF, Martin JF, Colin JF, Bertin F, Cayron C (2011) Confirmation of the domino-cascade model by LiFePO4/FePO4 precession electron diffraction. Chem Mater 23(20):4515–4524
Chueh WC, Gabaly FE, Sugar JD, Bartelt NC, McDaniel AH, Fenton KR, Zavadil KR, Tyliszczak T, Lai W, McCarty KF (2013) Intercalation pathway in many-particle LiFePO4 electrode revealed by nanoscale state-of-charge mapping. Nano Lett 13(3):866–872
Nelson Weker J, Li Y, Shanmugam R, Lai W, Chueh WC (2015) Tracking non-uniform mesoscale transport in LiFePO4 agglomerates during electrochemical cycling. ChemElectroChem 2(10):1576–1581
Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) Phospho-olivines as positive-electrode materials for rechargeable lithium batteries., J Electrochem Soc 144(4):1188–1194
Yamada A, Koizumi H, Sonoyama N, Kanno R (2005) Phase change in LixFePO4. Electrochem. Solid-State Lett 8(8):A409–A413
Srinivasan V, Newman J (2004) Discharge model for the lithium iron-phosphate electrode. J Electrochem Soc 151:A1517
Kasavajjula US, Wang C, Arce PE (2008) Discharge model for LiFePO4 accounting for the solid solution range., J Electrochem Soc 155(11):A866–A874
Dargaville S, Farrell TW (2010) Predicting active material utilization in LiFePO4 electrodes using a multiscale mathematical model. J Electrochem Soc 157(7):A830–A840
Singh GK, Ceder G, Bazant MZ (2008) Intercalation dynamics in rechargeable battery materials: general theory and phase-transformation waves in LiFePO4. Electrochim Acta 53(26):7599–7613
Burch D, Singh G, Ceder G, Bazant MZ (2008) Phase-transformation wave dynamics in LiFePO4. Solid State Phenom 139:95–100
Burch D, Bazant MZ (2009) Size-dependent spinodal and miscibility gaps for intercalation in nanoparticles. Nano Lett 9(11):3795–3800
Delmas C, Maccario M, Croguennec L, Le Cras F, Weill F (2008) Lithium deintercalation in LiFePO4 nanoparticles via a domino-cascade model., Nat Mater 7(8):665–671
Sasaki T, Ukyo Y, Novák P (2013) Memory effect in a lithium-ion battery. Nat Mater 12(6):569–575
Thomas-Alyea KE (2008) Modeling resistive-reactant and phase-change materials in battery electrodes., ECS Trans 16(13):155–165
Safari M, Delacourt C (2011) Mathematical modeling of lithium iron phosphate electrode: galvanostatic charge/discharge and path dependence. J Electrochem Soc 158:A63
Safari M, Delacourt C (2011) Modeling of a commercial graphite/LiFePO[sub 4] Cell. J Electrochem Soc 158:A562–A571
Thorat IV (2009) Understanding performance-limiting mechanisms in Li-ion batteries for high-rate applications. Brigham Young University, ProQuest Dissertations Publishing
Farkhondeh M, Delacourt C (2012) Mathematical modeling of commercial LiFePO4 electrodes based on variable solid-state diffusivity. J Electrochem Soc 159(2):A177–A192
Farkhondeh M, Safari M, Pritzker M, Fowler M, Han T, Wang J, Delacourt C (2014) Full-range simulation of a commercial LiFePO4 electrode accounting for bulk and surface effects: a comparative analysis. J Electrochem Soc 161:A201
Andersson AS, Thomas JO (2001) The source of first-cycle capacity loss in LiFePO4. J Power Sources 97:498–502
Dreyer W, Jamnik J, Guhlke C, Huth R, Moškon J, Gaberšček M (2010) The thermodynamic origin of hysteresis in insertion batteries. Nat Mater 9(5):448–453
Dreyer W, Guhlke C, Herrmann M (2011) Hysteresis and phase transition in many-particle storage systems. Continuum Mech Thermodyn 23, 3:211–231
Farkhondeh M, Pritzker M, Fowler M, Safari M, Delacourt C (2014) Mesoscopic modeling of Li insertion in phase-separating electrode materials: application to lithium iron phosphate. Phys Chem Chem Phys 16(41):22555–22565
Farkhondeh M, Pritzker M, Fowler M, Delacourt C (2017) Mesoscopic modeling of a LiFePO4 electrode: experimental validation under continuous and intermittent operating conditions. J Electrochem Soc 164(11):E3040–E3053
Wang J, Sun X (2015) Olivine LiFePO4: the remaining challenges for future energy storage. Energy Environ Sci 8(4):1110–1138
Doyle M, Fuller M, Newman J (1993) Modeling of galvanostatic charge and discharge of the lithium/polymer/insertion cell. J Electrochem Soc 140(6):1526–1533
Delacourt C, Safari M (2011) Analysis of lithium deinsertion/insertion in LiyFePO4 with a simple mathematical model. Electrochim Acta 56(14):5222–5229
Maheshwari A, Dumitrescu MA, Destro M, Santarelli M (2016) Inverse parameter determination in the development of an optimized lithium iron phosphate-Graphite battery discharge model. J Power Sources 307:160–172
Prada E, Di Domenico D, Creff Y, Bernard J, Sauvant-Moynot V, Huet F (2012) Simplified electrochemical and thermal model of LiFePO4-graphite Li-ion batteries for fast charge applications. J Electrochem Soc 159:A1508–A1519
Jokar A, Rajabloo B, Désilets M, Lacroix M, An inverse method for estimating the electrochemical parameters of lithium-ion batteries, part A: methodology (2016) J Electrochem Soc 163(14):A2876-A2886
Rajabloo B, Jokar A, Désilets M, Lacroix M (2016) An inverse method for estimating the electrochemical parameters of lithium-ion batteries, Part II: implementation, J Electrochem Soc. https://doi.org/10.1149/2.0221702jes
Delacourt C, Laffont L, Bouchet R, Wurm C, Leriche JB, Morcrette M, Tarascon JM, Masquelier C (2005) Toward understanding of electrical limitations (electronic, ionic) in LiMPO4 (M = Fe, Mn) electrode materials. J Electrochem Soc 152(5):A913–A921
Dominko R, Gaberscek M, Drofenik J, Bele M, Pejovnik S, Jamnik J (2003) The role of carbon black distribution in cathodes for Li ion batteries. J Power Sources 119:770–773
Marcicki J (2012) Modeling, parametrization, and diagnostics for lithium-ion batteries with automotive applications, Dissertation, The Ohio State University
Santhanagopalan S, Guo Q, Ramadass P, White RE (2006) Review of models for predicting the cycling performance of lithium ion batteries. J Power Sources 156(2):620–628
Doyle M, Newman J, Gozdz AS, Schmutz CN, Tarascon J (1996) Comparison of modeling predictions with experimental data from plastic lithium ion cells., J Electrochem Soc 143(6):1890–1903
Fuller TF, Doyle M, Newman J (1994) Relaxation phenomena in lithium-ion-insertion cells. J Electrochem Soc 141(4):982–990
Atlung S, West K, Jacobsen T (1979) Dynamic aspects of solid solution cathodes for electrochemical power sources., J Electrochem Soc 126(8):1311–1321
Haran BS, Popov BN, White RE (1998) Determination of the hydrogen diffusion coefficient in metal hydrides by impedance spectroscopy., J Power Sources 75(1):56–63
Guo M, Sikha G, White RE (2011) Single-particle model for a lithium-ion cell: thermal behavior., J Electrochem Soc 158(2):A122–A132
Arora P, Doyle M, Gozdz AS, White RE, Newman J (2000) Comparison between computer simulations and experimental data for high-rate discharges of plastic lithium-ion batteries., J Power Sources 88(2):219–231
Paxton B, Newman J (1996) Variable diffusivity in intercalation materials a theoretical approach. J Electrochem Soc 143(4):1287–1292
Acknowledgements
The authors are very grateful to Hydro-Québec and to the Natural Sciences and Engineering Council of Canada (NSERC) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rajabloo, B., Jokar, A., Wakem, W. et al. Lithium iron phosphate electrode semi-empirical performance model. J Appl Electrochem 48, 663–674 (2018). https://doi.org/10.1007/s10800-018-1189-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-018-1189-z