Abstract
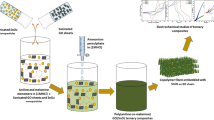
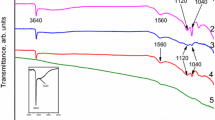
In this paper, graphite nanosheets were modified by aminopyrene (Amin), 1-pyrenecarboxylic acid (PCA) and cetyltrimethyl ammonium bromide. Based on physical (field-emission scanning electron microscopy, high-resolution transmission electron microscopy, X-ray diffraction, X-ray photoelectron spectroscopy, and Raman spectroscopy) and electrochemical tests (cyclic voltammetry, electrochemical impedance spectroscopy and galvanostatic charging/discharging curves), their physical and chemical properties were investigated. The physical characterization and electrochemical performance analysis indicated that the different reducing agents, technical methods and small organic molecules significantly influenced the precipitation of MnO2. When the small organic molecule was PCA, the G/MnO2 composite material had the best electrochemical capacity and stability. PCA possesses four rings; therefore, PCA can interact with graphite layers and peel off the graphite to form MnO2/single-layer graphene. During the loading of MnO2, PCA did not influence the structure of MnO2 crystals. After modification by PCA, the MnO2/C composite possessed long-term cycling stability. The changes in the morphological and structural evolutions in nanocrystalline MnO2 for the MnO2/C composite differed from those observed for pure MnO2.
Graphical Abstract










Similar content being viewed by others
References
Wang G, Xu H, Lu L, Zhao H, Tian H, An W (2016) High-voltage asymmetric supercapacitor based on MnO2 nanotubes//active carbon-multiwalled carbon nanotubes. J Appl Electrochem 46:1091–1097
Lv Y, Che H, Liu A, Mu J, Dai C, Zhang X, Bai Y, Wang G, Zhang Z (2017) Urchin-like α-FeOOH@MnO2 core–shell hollow microspheres for high-performance supercapacitor electrode. J Appl Electrochem 47:433–444
Aref A, Tang Y (2014) Chemical bath deposition synthesis and electrochemical properties of MnO2 thin film: effect of deposition time and bath temperature. Mater Sci-Poland 32:555–564
Lu X, Dou H, Zhang X (2016) Mesoporous carbon nanospheres inserting into graphene sheets for flexible supercapacitor film electrode. Mater Lett 178:304–307
Rajagopalan B, Hur SH, Chung JS (2015) Surfactant-treated graphene covered polyaniline nanowires for supercapacitor electrode. Nanoscale Res Lett 10:183–183
Xu Y, Lin Z, Huang X, Liu Y, Huang Y, Duan X (2013) Flexible solid-state supercapacitors based on three-dimensional graphene hydrogel films. ACS Nano 7:4042–4049
Shumba M, Centane S, Chindeka F, Nyokong T (2017) Nanocomposites of sulphur-nitrogen co-doped graphene oxide nanosheets and cobalt mono carboxyphenoxy phthalocyanines for facile electrocatalysis. J Electroanal Chem 791:36–48
Lee S, Jin X, Kim IY, Gu TH, Choi JW, Nahm S, Hwang SJ (2016) Superior additive of exfoliated RuO2 nanosheet for optimizing the electrode performance of metal oxide over graphene. J Phys Chem C 120:11786–11796
Zhou H, Zhai HJ (2016) Rapid preparation of the hybrid of MnO2 dispersed on graphene nanosheets with enhanced supercapacitive performance. Electrochim Acta 215:339–345
Liu H, Hu Z, Tian L, Su Y, Ruan H, Zhang L, Hu R (2016) Reduced graphene oxide anchored with δ-MnO2 nanoscrolls as anode materials for enhanced Li-ion storage. Ceram Int 42:13519–13524
Wu ZS, Winter A, Chen L, Sun Y, Turchanin A, Feng X, Mullen K (2012) Three-dimensional nitrogen and boron co-doped graphene for high-performance all-solid-state supercapacitors. Adv Mater 24:5130–5135
Zhou J, Lian J, Hou L, Zhang J, Gou H, Xia M, Zhao Y, Strobel TA, Tao L, Gao F (2015) Ultrahigh volumetric capacitance and cyclic stability of fluorine and nitrogen co-doped carbon microspheres. Nat Commun 6:8503–8503
Wang Y, Zhitomirsky I (2011) Cathodic electrodeposition of Ag-doped manganese dioxide films for electrodes of electrochemical supercapacitors. Mater Lett 65:1759–1761
Conway BE (1999) Electrochemical supercapacitors—scientific fundamentals and technological applications. Kluwer Academic/Plenum Publishers, New York
Chen Y, Hu W, Gan H, Wang JW, Shi XC (2017) Enhancing high-rate capability of MnO2 film electrodeposited on carbon fibers via hydrothermal treatment. Electrochim Acta 246:890–896
Zhu Y, Li Z, Huang C, Wang Y (2015) Effect of the number of benzene-ring, the functional groups and the absorbent material on the performance of Pt nanoparticles supported on modified graphite nanoplatelet. Electrochim Acta 153:439–447
Yan L, Zheng YB, Zhao F, Li S, Gao X, Xu B, Weiss PS, Zhao Y (2012) Chemistry and physics of a single atomic layer: strategies and challenges for functionalization of graphene and graphene-based materials. Chem Soc Rev 41:97–114
Zhao B, Zhuang H, Fang T, Jiao Z, Liu R, Ling X, Lu B, Jiang Y (2014) Self-assembly of NiO/graphene with three-dimension hierarchical structure as high-performance electrode material for supercapacitors. J Alloy Compd 597:291–298
Ghaemi M, Ataherian F, Zolfaghari A, Jafari SM (2008) Charge storage mechanism of sonochemically prepared MnO2 as supercapacitor electrode: effects of physisorbed water and proton conduction. Electrochim Acta 53:4607–4614
Zolfaghari A, Ataherian F, Ghaemi M, Gholami A (2007) Capacitive behavior of nanostructured MnO2 prepared by sonochemistry method. Electrochim Acta 52:2806–2814
Hamid RN, Parviz N, Mohammad RG (2016) Electrochemical study of a novel high-performance supercapacitor based on MnO2/nitrogen-doped graphene nanocomposite. Appl Surf Sci 366:552–560
Tsai I-L, Cao J, Fevre LL, Wang B, Todd R, Dryfe RA, Forsyth AJ (2017) Graphene-enhanced electrodes for scalable supercapacitors. Electrochim Acta 257:372–379
An X, Simmons T, Shah R, Wolfe C, Lewis KM, Washington M, Nayak SK, Talapatra S, Kar S (2010) Stable aqueous dispersions of noncovalently functionalized graphene from graphite and their multifunctional high-performance applications. Nano Lett 10:4295–4301
Sekar P, Anothumakkool B, Kurungot S (2015) 3D polyaniline porous layer anchored pillared graphene sheets: enhanced interface joined with high conductivity for better charge storage applications. ACS Appl Mater Interfaces 7:7661–7669
Ghodbane O, Pascal JL, Favier F (2009) Microstructural effects on charge-storage properties in MnO2-based electrochemical supercapacitors. ACS Appl Mater Interfaces 1:1130–1139
Zhao S, Liu T, Javed MS, Zeng W, Hussain S, Zhang Y, Peng X (2016) Rational synthesis of Cu-doped porous d-MnO2 microsphere for high performance supercapacitor applications. Electrochim Acta 191:716–723
Li J, Qu Z, Qin Y, Wang H (2016) Effect of MnO2 morphology on the catalytic oxidation of toluene over Ag/MnO2 catalysts. Appl Surf Sci 385:234–240
Mallakpour S, Madani M (2016) Functionalized-MnO2/chitosan nanocomposites: a promising adsorbent for the removal of lead ions. Carbohydr Polym 147:53–59
Cetinkaya T, Akbulut H, Tokur M, Ozcan S, Uysal M (2016) High capacity graphene/α-MnO2 nanocomposite cathodes for Li–O2 batteries. Int J Hydrogen Energy 41:9746–9754
Zhang N, Fu C, Liu D, Li Y, Zhou H, Kuang Y (2016) Three-dimensional pompon-like MnO2/graphene hydrogel composite for supercapacitor. Electrochim Acta 210:804–811
Ning P, Duan X, Ju X, Lin X, Tong X, Pan X, Wang T, Li Q (2016) Facile synthesis of carbon nanofibers/MnO2 nanosheets as high-performance electrodes for asymmetric supercapacitors. Electrochim Acta 210:754–761
Bello A, Fashedemi OO, Barzegar F, Madito MJ, Momodu DY, Masikhwa TM, Dangbegnon JK, Manyala N (2016) Microwave synthesis: characterization and electrochemical properties of amorphous activated carbon-MnO2 nanocomposite electrodes. J Alloys Compd 681:293–300
Chen S, Zhu J, Wang X (2010) From graphene to metal oxide nanolamellas: a phenomenon of morphology transmission. ACS Nano 4:6212–6218
Chigane M, Ishikawa M, Izaki M (2001) Preparation of manganese oxide thin films by electrolysis/chemical deposition and electrochromism. J Electrochem Soc 148:D96–D101
Wang B, Qiu J, Feng H, Wang N, Sakai E, Komiyama T (2016) Preparation of MnO2/carbon nanowires composites for supercapacitors. Electrochim Acta 212:710–721
Yang M, Choi BG (2016) Rapid one-step synthesis of conductive and porous MnO2/graphene nanocomposite for high performance supercapacitors. J Electroanal Chem 776:134–138
Ma Z, Zhao T (2016) Reduced graphene oxide anchored with MnO2 nanorods as anode for high rate and long cycle lithium ion batteries. Electrochim Acta 201:165–171
Vinny RT, Chaitra K, Venkatesh K, Nagaraju N, Kathyayini N (2016) An excellent cycle performance of asymmetric supercapacitor based on bristles like α-MnO2 nanoparticles grown on multiwalled carbon nanotubes. J Power Sources 309:212–220
Wang Z, Qin Q, Xu W, Yan J, Wu Y (2016) Long cyclic life in manganese oxide-based electrodes. ACS Appl Mater Interface 8:18078–18088
Yuan L, Liu X, Xiao X, Zhai T, Dai J, Zhang F, Hu B, Wang X, Gong L, Chen J, Hu C, Tong Y, Zhou J, Wang ZL (2012) Flexible solid-state supercapacitors based on carbon nanoparticles/MnO2 nanorods hybrid structure. ACS Nano 6:656–661
Ataherian F, Lee KT, Wu NL (2010) Long-term electrochemical behaviors of manganese oxide aqueous electrochemical capacitor under reducing potentials. Electrochim Acta 55:7429–7435
Acknowledgements
Financial support for this work was provided by Tianjin Natural Science Foundation (Grant Nos. 16JCYBJC21100 and 16JCQNJC06000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, Y., Jiao, W. & Huang, C. Effect of the noncovalent functionalization of graphite nanoflakes on the performance of MnO2/C composites. J Appl Electrochem 48, 187–199 (2018). https://doi.org/10.1007/s10800-018-1151-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-018-1151-0