Abstract
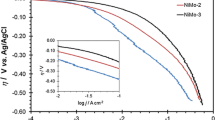
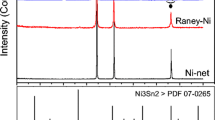
Platinum is the electrode material with the highest catalytic activity for the hydrogen evolution reaction (HER). However, its high cost and scarcity are the two major barriers for its usage in the industrial alkaline water electrolysis, which requires searching for other cheaper and more available materials with good catalytic activity. Ni-based materials have attracted more and more attention due to their good activity for the HER and sufficient corrosion resistance in alkaline solutions at considerable low cost. According to the Brewer intermetallic bonding theory, molybdenum alloyed with nickel (hypo–hyper-d-electronic transition metal) could improve the intrinsic catalytic activity for the HER. In this work, Ni and NiMo metallic coatings were galvanostatically electrodeposited on a stainless steel AISI 304 substrate by means of the double-template electrochemical process. The evaluation of these electrodes as H2-evolving cathodes was done in 30 % wt. KOH by pseudo-steady-state polarization curves and electrochemical impedance spectroscopy (EIS) at different temperatures. From Tafel curves results, it is shown that the NiMo electrodes have higher catalytic activity than Ni. On the other hand, from EIS results, it is possible to conclude that the NiMo electrodes showed higher intrinsic catalytic activity for HER than the pure Ni electrode as a consequence of alloying hypo–hyper-d-electronic transition metals.
Graphical Abstract









Similar content being viewed by others
References
Verizoglu TN, Sherif SA, Barbir F (2005) Hydrogen energy solutions. In: Agardy FJ, Nemerow NL (eds) Enviromental solutions. Elsevier Inc., USA, pp 143–180
Barbir F (2009) Transition to renewable energy systems with hydrogen as an energy carrier. Energy 34:308–312. doi:10.1016/j.energy.2008.07.007
Veziroglu TN, Barbir F (1992) Hydrogen: the wonder fuel. Int J Hydrog Energy 17:391–404. doi:10.1016/0360-3199(92)90183-W
Zeng K, Zhang D (2010) Recent progress in alkaline water electrolysis for hydrogen production and applications. Progr Energy Combust 36:307–326. doi:10.1016/j.pecs.2009.11.002
Stojić DL, Marĉeta MP, Sovilj SP, Miljanić SS (2003) Hydrogen generation from water electrolysis—possibilities of energy saving. J Power Sources 118:315–319. doi:10.1016/S0378-7753(03)00077-6
Souza RF, Padilha JC, Gonçalves RS, Souza MO, Rault-Berthelot J (2007) Electrochemical hydrogen production from water electrolysis using ionic liquid as electrolytes: towards the best device. J Power Sources 164:792–798. doi:10.1016/j.jpowsour.2006.11.049
Stojić DL, Grozdić TD, Kaninski MPM, Maksić AD, Simić ND (2006) Intermetallics as advanced cathode materials in hydrogen production via electrolysis. Int J Hydrog Energy 31:841–846. doi:10.1016/j.ijhydene.2005.08.009
Yazici B, Tatli G, Galip H, Erbil M (1995) Investigation of suitable cathodes for the production of hydrogen gas by electrolysis. Int J Hydrog Energy 20:957–965. doi:10.1016/0360-3199(95)00032-9
Solmaz R, Kardaş G (2011) Fabrication and characterization of NiCoZn–M (M: Ag, Pd and Pt) electrocatalysts as cathode materials for electrochemical hydrogen production. Int J Hydrog Energy 36:12079–12087. doi:10.1016/j.ijhydene.2011.06.101
Lasia A (2003) Hydrogen evolution reaction. In: Vielstich W, Lamm A, Gasteiger HA (eds.) Handbook of fuel cells: fundamentals, technology and applications. Volume 2: Fuel cell electrocatalysis, John Wiley and Sons Ltd, Chichester, pp 416–440
Lasia A, Rami A (1990) Kinetics of hydrogen evolution on nickel electrodes. J Electroanal Chem Interfacial Electrochem 294:123–141. doi:10.1016/0022-0728(90)87140-F
Rami A, Lasia A (1992) Kinetics of hydrogen evolution on Ni–Al alloy electrodes. J Appl Electrochem 22:376–382. doi:10.1007/BF01092692
Chen LL, Lasia A (1992) Study of the kinetics of hydrogen evolution reaction on Nickel–Zinc powder electrodes. J Electrochem Soc 139:3214–3219. doi:10.1149/1.2069055
Herraiz-Cardona I, Ortega E, García-Antón J, Pérez-Herranz V (2011) Assessment of the roughness factor effect and the intrinsic catalytic activity for hydrogen evolution reaction on Ni-based electrodeposits. Int J Hydrog Energy 36:9428–9438. doi:10.1016/j.ijhydene.2011.05.047
Lupi C, Dell’Era A, Pasquali M (2009) Nickel–cobalt electrodeposited alloys for hydrogen evolution in alkaline media. Int J Hydrogen Energy 34:2101–2106. doi:10.1016/j.ijhydene.2009.01.015
Herraiz-Cardona I, Ortega E, Pérez-Herranz V (2011) Impedance study of hydrogen evolution on Ni/Zn and Ni–Co/Zn stainless steel based electrodeposits. Electrochim Acta 56:1308–1315. doi:10.1016/j.electacta.2010.10.093
Herraiz-Cardona I, González-Buch C, Valero-Vidal C, Ortega E, Pérez-Herranz V (2013) Co-modification of Ni-based type Raney electrodeposits for hydrogen evolution reaction in alkaline media. J Power Sources 240:698–704. doi:10.1016/j.jpowsour.2013.05.041
Herraiz-Cardona I, Ortega E, Vázquez-Gómez L, Pérez-Herranz V (2011) Electrochemical characterization of a NiCo/Zn cathode for hydrogen generation. Int J Hydrog Energy 36:11578–11587. doi:10.1016/j.ijhydene.2011.06.067
Navarro-Flores E, Chong Z, Omanovic S (2005) Characterization of Ni, NiMo, NiW and NiFe electroactive coatings as electrocatalysts for hydrogen evolution in an acidic medium. J Mol Catal A-Chem 226:179–197. doi:10.1016/j.molcata.2004.10.029
Giz MJ, Benito SC, González ER (2000) NiFeZn codeposited as a cathode material for the production of hydrogen by water. Int J Hydrog Energy 25:621–626. doi:10.1016/S0360-3199(99)00084-1
Solmaz R, Kardaş G (2009) Electrochemical deposition and characterization of NiFe coatings as electrocatalytic materials for alkaline water electrolysis. Electrochim Acta 54:3726–3734. doi:10.1016/j.electacta.2009.01.064
Ullal Y, Hegde AC (2014) Electrodeposition and electro-catalytic study of nanocrystalline Ni–Fe alloy. Int J Hydrog Energy 39:10485–10492. doi:10.1016/j.ijhydene.2014.05.016
Fan C, Piron DL, Sleb A, Paradis P (1994) Study of electrodeposited nickel–molybdenum, nickel–tungsten, cobalt–molybdenum, and cobalt–tungsten as hydrogen electrodes in alkaline water electrolysis. J Electrochem Soc 141:382–387. doi:10.1149/1.2054736
Solmaz R, Döner A, Kardaş G (2009) The stability of hydrogen evolution activity and corrosion behavior of NiCu coatings with long-term electrolysis in alkaline solution. Int J Hydrog Energy 34:2089–2094. doi:10.1016/j.ijhydene.2009.01.007
Wu L, He Y, Lei T, Nan B, Xu N, Zou J, Huang B, Liu CT (2014) The stability of hydrogen evolution activity and corrosion behavior of porous Ni3Al–Mo electrode in alkaline solution during long-term electrolysis. Energy 67:19–26. doi:10.1016/j.energy.2014.02.033
Sheela G, Pushpavanam M, Pushpavanam S (2002) Zinc–nickel alloy electrodeposits for water electrolysis. Int J Hydrog Energy 27:627–633. doi:10.1016/S0360-3199(01)00170-7
Solmaz R, Kardaş G (2007) Hydrogen evolution and corrosion performance of NiZn coatings. Energy Convers Manag 48(2007):583–591. doi:10.1016/j.enconman.2006.06.004
Brewer L (1963) In: Beck PA (ed) Electronic structure and alloy chemistry of transition elements. Interscience, New York, p 221
Jaksic MM (1984) Electrocatalysis of hydrogen evolution in the light of the brewer—engel theory for bonding in metals and intermetallic phases. Electrochim Acta 29:1539–1550. doi:10.1016/0013-4686(84)85007-0
Jaksic MM, Jaksic JM (1984) Fermi dynamics and some structural bonding aspects of electrocatalysis for hydrogen evolution. Electrochim Acta 39:1695–1714. doi:10.1016/0013-4686(94)85155-7
Jaksic MM, Lacnjevac CM, Grgur BN, Krstajic NV (2000) Volcano plots along intermetallic hypo–hyper-d-electronic phase diagrams and electrocatalysis for hydrogen electrode reactions. J New Mat Electrochem Systems 3:131–144
Jaksic MM (2001) Hypo–hyper-d-electronic interactive nature of interionic synergism in catalysis and electrocatalysis for hydrogen reactions. Int J Hydrog Energy 26:559–578. doi:10.1016/S0360-3199(00)00120-8
Raj IA, Venkatesan VK (1988) Characterization of nickel–molybdenum and nickel–molybdenum–iron alloy coatings as cathodes for alkaline water electrolysers. Int J Hydrog Energy 13:215–223. doi:10.1016/0360-3199(88)90088-2
Gennero de Chialvo MR, Chialvo AC (1998) Hydrogen evolution reaction on smooth Ni(1 − x) + Mo(x) alloys (0 ≤ x ≤ 0.25). J Electroanal Chem 448:87–93. doi:10.1016/S0022-0728(98)00011-4
Simpraga R, Bai L, Conway BE (1995) Real area and electrocatalysis factors in hydrogen evolution kinetics at electrodeposited Ni–Mo and Ni–Mo–Cd composites: effect of Cd content and nature of substrate. J Appl Electrochem 25:628–641. doi:10.1007/BF00241924
Fan C, Piron DL, Paradis P (1994) Hydrogen evolution on electrodeposited nickel–cobalt–molybdenum in alkaline water electrolysis. Electrochim Acta 39:2715–2722. doi:10.1016/0013-4686(94)00263-0
Raj IA, Vasu KI (1990) Transition metal-based hydrogen electrodes in alkaline solution—electrocatalysis on nickel based binary alloy coatings. J Appl Electrochem 20:32–38. doi:10.1007/BF01012468
Raj IA, Vasu KI (1992) Transition metal-based cathodes for hydrogen evolution in alkaline solution: electrocatalysis on nickel-based ternary electrolytic codeposits. J Appl Electrochem 22:471–477. doi:10.1007/BF01077551
Divisek J, Schmitz H, Balej J (1989) Ni and Mo coatings as hydrogen cathodes. J Appl Electrochem 19:519–530. doi:10.1007/BF01022108
Huot JY, Trudeau ML, Schulz R (1991) Low hydrogen overpotential nanocrystalline Ni–Mo cathodes for alkaline water electrolysis. J Electrochem Soc 138:1316–1321. doi:10.1149/1.2085778
Krstajić NV, Jović VD, Lj Gajić-Krstajić, Jović BM, Antozzi AL, Martelli GN (2008) Electrodeposition of Ni–Mo alloy coatings and their characterization as cathodes for hydrogen evolution in sodium hydroxide solution. Int J Hydrog Energy 33:3676–3687. doi:10.1016/j.ijhydene.2008.04.039
Dini JW (1993) Electrodeposition: the materials science of coating and substances. Noyes Publications, New Jersey
Wood D (1938) Metal Industry 36:330
Herraiz-Cardona I, Ortega E, Vázquez-Gómez L, Pérez-Herranz V (2012) Double-template fabrication of three-dimensional porous nickel electrodes for hydrogen evolution reaction. Int J Hydrog Energy 37:2147–2156. doi:10.1016/j.ijhydene.2011.09.155
Herraiz-Cardona I, González-Buch C, Ortega E, García-Antón J, Pérez-Herranz V (2013) Energy efficiency improvement of alkaline water electrolysis by using 3D Ni cathodes fabricated via a double-template electrochemical process. Chem Eng Trans 32:451–456. doi:10.3303/CET1332076
Shin H, Liu M (2004) Copper foam structures with highly porous nanostructured walls. Chem Mater 16:5460–5464. doi:10.1021/cm048887b
Bonou L, Eyraud M, Denoyel R, Massiani Y (2002) Influence of additives on Cu electrodeposition mechanisms in acid solution: direct current study supported by non-electrochemical measurements. Electrochim Acta 47:4139–4148. doi:10.1016/S0013-4686(02)00356-0
Nagy Z, Blaudeau JP, Hung NC, Curtiss LA, Zurawski DJ (1995) Chloride ion catalysis of the copper deposition reaction. J Electrochem Soc 142:L87–L89. doi:10.1149/1.2044254
Soares DM, Wasle S, Weil KG, Doblhofer K (2002) Chloride ion catalysis of the copper deposition reaction. J Electroanal Chem 532:353–358
Halim J, Abdel-Karim R, El-Raghy S, Nabil M, Waheed A (2012) Electrodeposition and characterization of nanocrystalline Ni–Mo catalysts for hydrogen production. J Nanomater 2012:9. doi:10.1155/2012/845673
García-Antón J, Igual-Muñoz A, Guiñón JL, Pérez-Herranz V (2000) Horizontal electrochemical cell by the electro-optical analysis of electrochemical process. Spain P-200002526
Kubisztal J, Budniok A, Lasia A (2007) Study of the hydrogen evolution reaction on nickel-based composite coatings containing molybdenum powder. Int J Hydrog Energy 32:1211–1218
Jukic A, Piljac J, Meticoš-Hukovic M (2001) Electrocatalytic behavior of the Co33Zr67 metallic glass for hydrogen evolution. J Mol Catal A-Chem 166:293–302. doi:10.1016/S1381-1169(00)00452-0
Savadogo O, Ndzebet E (2001) Influence of SiW12O40 4− on the electrocatalytic behaviour of Pt–Co alloy supported on carbon for water electrolysis in 3 M KOH aqueous solution. Int J Hydrog Energy 26:213–218. doi:10.1016/S0360-3199(00)00059-8
Correia AN, Machado SAS (1998) Hydrogen evolution on electrodeposited Ni and Hg ultramicroelectrodes. Electrochim Acta 43:367–373. doi:10.1016/S0013-4686(97)00050-9
Machado SAS, Avaca LA (1994) The hydrogen evolution reaction on nickel surfaces stabilized by H-absorption. Electrochim Acta 39:1385–1391. doi:10.1016/0013-4686(94)E0003-I
Tie-chui Y, Rui-di L, Ke-chao Z (2004) Electrocatalytic properties of Ni–S–Co coating electrode for hydrogen evolution in alkaline medium. Trans Nonferrous Metals Soc China 17:762–765. doi:10.1016/S1003-6326(07)60170-8
Tasic GS, Maslovara SP, Zugic DL, Maksic AD, Kaninski MPM (2011) Characterization of the Ni–Mo catalyst formed in situ during hydrogen generation from alkaline water electrolysis. Int J Hydrog Energy 36:11588–11595. doi:10.1016/j.ijhydene.2011.06.081
Bard AJ, Inzelt G, Scholz F (2008) Electrochemical dictionary, 1st edn. Springer, Berlin
Zeng K, Zhang D (2014) Evaluating the effect of surface modifications on Ni based electrodes for alkaline water electrolysis. Fuel 116:692–698. doi:10.1016/j.fuel.2013.08.070
Keyser H, Beccu KD, Gutjahr MA (1976) Abschätzung der porenstruktur poröser elektroden aus impedanzmessungen. Electrochim Acta 21:539–543
Levie R (1967) Electrochemical responses of porous and rough electrodes. In: Delahay P (ed) Advances in electrochemistry and electrochemical engineering, vol 6. Interscience, New York, pp 329–397
Brug GJ, Van den Eeden ALG, Sluyters-Rehbach M, Sluyters JH (1984) The analysis of electrode impedances complicated by the presence of a constant phase element. J Electroanal Chem 176:275–295
Trasatti S, Petrii OA (1991) Real surface area measurements in electrochemistry. Pure Appl Chem 63:711–734. doi:10.1351/pac199163050711
Chen L, Lasia A (1991) Study of the kinetics of hydrogen evolution reaction on nickel–zinc alloy electrodes. J Electrochem Soc 138:3321–3328. doi:10.1149/1.2085409
Kellenberger A, Vaszilcsin N, Brandl W, Duteanu N (2007) Kinetics of hydrogen evolution reaction on skeleton nickel and nickel–titanium electrodes obtained by thermal arc spraying technique. Int J Hydrog Energy 32:3258–3265. doi:10.1016/j.ijhydene.2007.02.028
Acknowledgments
The authors acknowledge the support of Generalitat Valenciana (PROMETEO/2010/023) and Universidad Politécnica de Valencia (PAID-06-10-2227). We wish to thank the Electron Microscopy Service of the UPV.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González-Buch, C., Herraiz-Cardona, I., Ortega, E. et al. Study of the catalytic activity of 3D macroporous Ni and NiMo cathodes for hydrogen production by alkaline water electrolysis. J Appl Electrochem 46, 791–803 (2016). https://doi.org/10.1007/s10800-016-0970-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-016-0970-0