Abstract
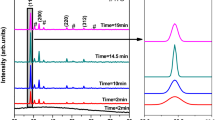
Film electrodes of a ternary metal oxide, bismuth copper vanadate (BiCu2VO6), were fabricated directly on FTO (fluorine-doped tin oxide glass) substrates by a chemical solution method using precursor solution of the component metal ions. To improve the purity of BiCu2VO6 films, temperature and time in pre-heating process were carefully selected by thermogravimetric and differential thermal analysis and X-ray photoelectron spectroscopy of the precursor film. The fabrication of pure BiCu2VO6 films keeping tight contact to the FTO substrate was confirmed by X-ray diffraction, cross-sectional scanning electron microscope images, and scratching the film using a soft paper. The band gap energy E g = 2.10 V and the flat band potential U FB = 0.43 V (vs. SHE at pH 0) for BiCu2VO6 were obtained. For the photoelectrode, wavelength dependence of the incident photon to current efficiency (IPCE) corresponded to the absorption spectrum, and the IPCE exhibited several-folds larger values than those of the electrode fabricated by depositing BiCu2VO6 particles on an FTO substrate.










Similar content being viewed by others
References
Walter M, Warren EL, McKone JR, Boettcher SW, Mi Q, Santori EA, Lewis NS (2010) Solar water splitting cells. Chem Rev 110:6446–6473
Chen X, Shen S, Guo L, Mao SS (2010) Semiconductor-based photocatalytic hydrogen generation. Chem Rev 110:6503–6570
Iwase A, Kato H, Kudo A (2013) The effect of Au cocatalyst loaded on La-doped NaTaO3 on photocatalytic water splitting and O2 photoreduction. Appl Catal B Environ 136–137:89–93
Porob DG, Maggard PA (2006) Flux syntheses of La-doped NaTaO3 and its photocatalytic activity. J Solid State Chem 179:1727–1732
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238:37–38
Hong SJ, Jun H, Borse PH, Lee JS (2009) Size effects of WO3 nanocrystals for photooxidation of water in particulate suspension and photoelectrochemical film systems. Int J Hydrog Energy 34:3234–3242
Kim ES, Nishimura N, Magesh G, Kim JY, Jang JW, Jun H, Kubota J, Domen K, Lee JS (2013) Fabrication of CaFe2O4/TaON heterojunction photoanode for photoelectrochemical water oxidation. J Am Chem Soc 135:5375–5383
Wang D, Li R, Zhu J, Shi J, Han J, Zong X, Li C (2012) Photocatalytic water oxidation on BiVO4 with the electrocatalyst as an oxidation cocatalyst: essential relations between electrocatalyst and photocatalyst. J Phys Chem C 116:5082–5089
Liu H, Nakamura R, Nakato Y (2005) Bismuth-copper vanadate BiCu2VO6 as a novel photocatalyst for efficient visible-light-driven oxygen evolution. ChemPhysChem 6:2499–2502
Chatchai P, Murakami Y, Kishioka SY, Nosaka AY, Nosaka Y (2008) FTO/SnO2/BiVO4 composite photoelectrode for water oxidation under visible light irradiation. Electrochem Solid-State Lett 11:H160–H163
Yagi M, Maruyama S, Sone K, Nagai K, Norimatsu T (2008) Preparation and photoelectrocatalytic activity of a nano-structured WO3 platelet film. J Solid State Chem 181:175–182
Nakabayashi Y, Nishikawa M, Nosaka Y (2014) Fabrication of CuBi2O4 photocathode through novel anodic electrodeposition for solar hydrogen production. Electrochim Acta 125:191–198
Qi JQ, Tian HY, Li LT, Chan HLW (2007) Fabrication of CuO nanoparticle interlinked microsphere cages by solution method. Nanoscale Res Lett 2:107–111
Osseo-Asara K, Mishra KK (1996) Solution chemical constraints in the chemical-mechanical polishing of copper: aqueous stability diagrams for the Cu-H2O and Cu-NH3-H2O systems. J Electron Mater 25(10):1599–1607
Jiang H, Endo H, Natori H, Nagai M, Kobayashi K (2008) Fabrication and photoactivities of spherical-shaped BiVO4 photocatalysts through solution combustion synthesis method. J Eur Ceram Soc 28:2955–2962
Smith RM, Martell AE (1989) Critical stability constant, 6th edn. Plenum Press, New York
Feick G (1954) The dissociation pressure and free energy of formation of ammonium nitrate. J Am Chem Soc 76(22):5858–5860
Tsuji E, Fukui K, Imanishi A (2014) Influence of surface roughening of rutile single-crystalline TiO2 on photocatalytic activity for oxygen photoevolution from water in acidic and alkaline solutions. J Phys Chem C 118:5406–5413
Min S, Wang F, Jin Z, Xu J (2014) Cu2O nanoparticles decorated BiVO4 as an effective visible-light-driven p-n heterojunction photocatalyst for methylene blue degradation. Super Lattices Microstruct 74:294–307
Reddy BM, Ganesh I, Reddy EP (1997) Study of dispersion and thermal stability of V2O5/TiO2-SiO2 catalysts by XPS and other techniques. J Phys Chem B 101:1769–1774
Yang R, Yang L, Tao T, Ma F, Xu M, Zhang Z (2014) Contrastive study of structure and photocatalytic performance with three-dimensionally ordered macroporous CuO–TiO2 and CuO/TiO2. Appl Surf Sci 288:363–368
Chatchai P, Kishioka S, Murakami Y, Nosaka A, Nosaka Y (2013) Photoelectrocatalytic performance of WO3/BiVO4 toward the dye degradation. Electrochim Acta 94:314–319
Imanishi A, Okamura T, Ohashi N, Nakamura R, Nakato Y (2007) Mechanism of water photooxidation reaction at atomically flat TiO2 (Rutile) (110) and (100) surfaces: dependence on solution pH. J Am Chem Soc 129:11569–11578
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakabayashi, Y., Nishikawa, M. & Nosaka, Y. Fabrication of bismuth copper vanadate electrodes through feasible chemical solution method for visible light-induced water oxidation. J Appl Electrochem 46, 9–16 (2016). https://doi.org/10.1007/s10800-015-0890-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-015-0890-4