Abstract
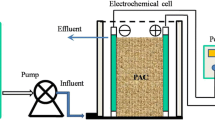
The electrochemical capacitance behavior of a packed bed electrochemical reactor (PBER) toward phenol electro-oxidation in aqueous solution was studied. In the absence of phenol, a decrease in the response current (i) coupled with an increase of the specific capacitance (C s ) was obtained for PBER compared with a flat-bed electrochemical reactor. However, following intercalation of phenol, simultaneous improvement of i, and reduction of C s were observed. The reasons for the former were ascribed to an increased resistance, an abundant pore structure for charge, and electrolyte transportation owing to the packing of the fixed granular active carbon (GAC) filler, whereas anode extension and formation of the polyoxyphenylene film on the anode and the GAC surface were responsible for the latter. Although significant attenuation (42.06 % after 300 cycles at current density 1.90 mA cm−2) of C s was observed for PBER in the long cycle test, the non-negligible C s value (30.86 mF cm−2) holds great promise for pulsating power supplies. An equivalent circuit associated with electrochemical capacitance and resistance distribution was proposed, which gives an exhaustive explanation of the electrical characteristics during the phenol electro-oxidation process.






Similar content being viewed by others
References
Nageswara RN, Rohit M, Gedam N, Parameswaran PN, Astik JK (2009) Kinetics of electrooxidation of landfill leachate in a three-dimensional carbon bed electrochemical reactor. Chemosphere 76:1206–1212. doi:10.1016/j.chemosphere.2009.06.009
Nageswara RN, Rohit M (2012) Efficient degradation of reactive blue 4 in carbon bed electrochemical reactor. Chem Eng J 184:23–32. doi:10.1016/j.cej.2011.12.014
Zhao X, Li AZ, Mao R, Liu HJ, Qu JH (2014) Electrochemical removal of haloacetic acids in a three dimensional electrochemical reactor with Pd-GAC particles as fixed filler and Pd-modified carbon paper as cathode. Water Res 51:134–143. doi:10.1016/j.watres.2013.12.028
Niu JF, Maharana D, Xu JL, Chai Z, Bao YP (2013) A high activity of Ti/SnO2-Sb electrode in the electrochemical degradation of 2, 4-dichlorophenol in aqueous solution.J. Environ Sci 25(1424–1430):1001. doi:10.1016/S-0742(12)60103-X
Zhang C, Jiang YH, Li YL, Hu ZX, Zhou L, Zhou MH (2013) Three-dimensional electrochemical process for wastewater treatment: a general review. Chem Eng J 228:455–467. doi:10.1016/j.cej.2013.05.033
Wang C, Huang YK, Zhao Q, Ji M (2014) Treatment of secondary effluent using a three-dimensional electrode system: COD removal, biotoxicity assessment, and disinfection effects. Chem Eng J 243:1–6. doi:10.1016/j.cej.2013.12.044
Tung CH, Shen SY, Chang JH, Hsu YM, Lai YC (2013) Treatment of real printing wastewater with an electrocatalytic process. Sep Purif Technol 117:131–136. doi:10.1016/j.seppur.2013.07.028
Zhang F, Li GM, Sheng Y, Hu HK, Wang H (2006) Preparation of Mn-Sn-Sb/γ-Al2O3 particle-electrodes for electrocatalytic oxidation of phenol wastewater. J Chin Chem Soc 64:235–239
Xu HQ, Liu XN, Wang YQ, Wang LH, Sun YM (2009) Electro-catalytic performance of composite oxide Sn-Sb-Mn/ceramic particle electrode system. Acta Phys Chim Sin 25:840–846. doi:10.3866/PKU.WHXB20090509
Wang LZ, Zhao YM, Fu JF (2008) The influence of TiO2 and aeration on the kinetics of electrochemical oxidation of phenol in packed bed reactor. J Hazard Mater 160:608–613. doi:10.1016/j.jhazmat.2008.03.034
Polcaro AM, Palmas S, Renoldi F, Mascia M (2000) Three-dimensional electrodes for the electrochemical combustion of organic pollutants. Electrochim Acta 46:389–394. doi:10.1016/s0013-4686(00)00596-X
Panizza M, Michaud PA, Cerisola G, Comninellis C (2001) Electrochemical treatment of wastewater containing organic pollutants on boron-doped diamond electrodes: predication of specific energy consumption and required electrode area. Electrochem Commun 3:336–339. doi:10.1016/S1388-2481(01)00166-7
Mascia M, Vacca A, Palmas S (2012) Fixed bed reactors with three dimensional electrodes for electrochemical treatment of waters for disinfection. Chem Eng J 211–212:479–487. doi:10.1016/j.cej.2012.09.091
Wang LZ, Li P, Yan Q (2012) Modeling approach to phenol oxidation by a sand-based packed-bed electrode system (SPBEs). Water Sci Technol 66:422–428. doi:10.2166/wat.2012.165
Kant R, Sarathbadu M, Srivastav S (2013) Effect of uncompensated solution resistance on quasireversible charge transfer at rough and finite fractal electrode. Electrochim Acta 95:237–245. doi:10.1016/j.electacta.2013.02.010
Kong LB, Lu C, Liu MC, Luo YC, Kang L, Li XH, Frank CW (2014) The specific capacitance of sol-gel synthesised spinel MnCo2O4 in an alkaline electrolyte. Electrochim Acta 115:22–27. doi:10.1016/j.electacta.2013.10.089
Li XW, Chai YZ, Zhang H, Wang GC, Feng X (2012) Synthesis of polyaniline/tin oxide hybrid and its improved electrochemical capacitance performance. Electrochim Acta 85:9–15. doi:10.1016/j.electacta.2012.07.124
Li XW, Li XH, Dai N, Wang GC, Wang Z (2010) Preparation and electrochemical capacitance performances of super-hydrophilic conducting polyaniline. J Power Sources 195(5417–5421):2010. doi:10.1016/j.jpowsour.03.034
Xie YB, Wang Y, Du HX (2013) Electrochemical capacitance perfprmance of titanium nitride nanoarray. Mater Sci Eng B-Solid 178:1443–1451. doi:10.1016/j.mseb.2013.09.005
Barbieri O, Hahn M, Herzog A, Kötz R (2005) Capacitance limits of high surface area activated carbons for double layer capacitors. Carbon 43:1303–1310. doi:10.1016/j.carbon.2005.01.001
Liu Y, Yan D, Li YH, Wu ZG, Zhou RF, Li SK, Feng JJ, Wang J, Yan PX, Geng ZR (2014) Manganese dioxide nanosheet arrays grown on graphene oxide as an advanced material material for supercapacitors. Electrochim Acta 117:528–533. doi:10.1016/j.electacta.2013.11.121
Liu TG, Pell WG, Conway BE (1999) Stages in the development of thick cobalt oxide films exhibiting reversible redox behavior and pseudocapacitance. Electrochim Acta 44:2829–2842. doi:10.1016/S0013-4686(99)0002-X
Grupioni AAF, Arashiro E, Lassali TAF (2002) Voltammetric characterization of an iridium oxide-based system: the pseudocapacitive nature of the Ir0.3Mn0.7O2 electrode. Electrochim Acta 48:407–418. doi:10.1016/S0013-4686(02)00686-2
Hu CC, Chang KH (2000) Cyclic voltammetric deposition of hydrous ruthenium oxide for electrochemical capacitors: effects of codepositing iridium oxide. Electrochim Acta 45:2685–2696. doi:10.1016/S0013-4686(00)00386-8
Yuan CZ, Gao B, Zhang XG (2007) Electrochemical capacitance of NiO/Ru0.35V0.65O2 asymmetric electrochemical capacitor. J Power Sources 173:606–612. doi:10.1016/j.jpowsour.2007.04.034
Lee JW, Ahn TB, Soundararajan D, Jang MK, Kim JD (2011) Non-aqueous approach to the preparation of reduced graphene oxide/α-Ni (OH) 2 hybrid composites and their high capacitance behavior. Chem Commun 2011(47):6305–6307
Rochefort D, Pont AL (2006) Pseudocapacitive behaviour of RuO2 in a proton exchange ionic liquid. Electrochem Commun 8:1539–1543. doi:10.1016/j.elecom.2006.06.032
Qu DY, Shi H (1998) Studies of activated carbons used in double-layer capacitors. J Power Sources 74:99–107. doi:10.1016/S0378-7753(98)00038-X
Wang KP, Teng HS (2006) The performance of electric double layer capacitors using particulate porous carbons derived from PAN fiber and phenol-formaldehyde resin. Carbon 44(3218–3225):2006. doi:10.1016/j.carbon.06.031
Jang JH, Han SJ, Hyeon TW, Seung MO (2003) Electrochemical capacitor performance of hydrous ruthenium oxide/mesoporous carbon composite electrodes. J Power Sources 123:79–85. doi:10.1016/S0378-7753(03)00459-2
Kumagai S, Sato M, Tashima D (2013) Electrical double-layer capacitance of micro- and mesoporous-activated carbon prepared from rice husk and beet sugar. Electrochim Acta 114:617–626. doi:10.1016/j.electacta.2012.10.060
Panić VV, Dekanski AB, Vidaković TR, Mišković VB, Javanović BŽ, Nikolić BŽ (2005) Oxidation of phenol on RuO2-TiO2-Ti anodes. J Solid State Electrochem 9:43–54. doi:10.1007/s10008-004-0559-0
Maznai HA, Conway BE (2001) Auto-inhibition effects in anodic oxidation of phenols for electrochemical wastewater purification. J Serb Chem Soc 66:765–784
Zhang YJ, Sun CT, Lu P, Li KY, Song SY, Xue DF (2012) Crystallization design of MnO2 towards better supercapacitance. CrystEngComm 14:5892–5897. doi:10.1039/C2E225610J
Zhua G, Deng LJ, Wang JF, Kang LP, Liu ZH (2012) The simple preparation of birnessite-type manganese oxide with flower-like microsphere morphology and its remarkable capacity retention. Mater Res Bull 47:3533–3537. doi:10.1016/j.materresbull.2012.06.065
Aboutalebi SH, Chidembo AT, Salari M, Konstantinov K, Wexler D, Liu HK, Dou SX (2011) Comparison of GO, GO/MWCNTs composite and MWCNTs as potential electrode materials for supercapacitors. Energy Environ Sci 4:1855–1865. doi:10.1039/C1EE01039E
Li L, Seng KH, Liu HK, Nevirkovets IP, Guo ZP (2013) Synthesis of Mn3O4-anchored graphene sheet nanocomposites via a facile, fast microwave hydrothermal method and their super capacitive behavior. Electrochim Acta 87:801–808. doi:10.1016/j.electacta.2012.08.127
Yang WL, Gao Z, Wang J, Wang B, Liu LH (2013) Hydrothermal synthesis of reduced graphene sheets/Fe2O3 nanorods composites and their enhanced electrochemical performance for supercapacitors. Solid State Sci 20:46–53
Acknowledgments
This work was supported by the National Natural Science Foundation of China (50908226, 51221462), the Jiangsu Provincial Research Foundation for Basic Research (BK2011224), the Fundamental Research Funds for the Central Universities (2013QNA20) and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, P., Zhao, Y., Wang, L. et al. Electrochemical capacitance behavior of a packed bed electrochemical reactor toward phenol electro-oxidation. J Appl Electrochem 45, 177–184 (2015). https://doi.org/10.1007/s10800-014-0760-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-014-0760-5