Abstract
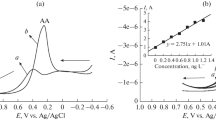
The performance of an amperometric biosensor constructed by associating tyrosinase (Tyr) enzyme with the advantages of a 3D gold nanoelectrode ensemble (GNEE) is evaluated in a flow-injection analysis (FIA) system for the analysis of l-dopa. GNEEs were fabricated by electroless deposition of the metal within the pores of polycarbonate track-etched membranes. A simple solvent etching procedure based on the solubility of polycarbonate membranes is adopted for the fabrication of the 3D GNEE. Afterward, enzyme was immobilized onto preformed self-assembled monolayers of cysteamine on the 3D GNEEs (GNEE-Tyr) via cross-linking with glutaraldehyde. The experimental conditions of the FIA system, such as the detection potential (−0.200 V vs. Ag/AgCl) and flow rates (1.0 mL min−1) were optimized. Analytical responses for l-dopa were obtained in a wide concentration range between 1 × 10−8 mol L−1 and 1 × 10−2 mol L−1. The limit of quantification was found to be 1 × 10−8 mol L−1 with a resultant % RSD of 7.23% (n = 5). The limit of detection was found to be 1 × 10−9 mol L−1 (S/N = 3). The common interfering compounds, namely glucose (10 mmol L−1), ascorbic acid (10 mmol L−1), and urea (10 mmol L−1), were studied. The recovery of l-dopa (1 × 10−7 mol L−1) from spiked urine samples was found to be 96%. Therefore, the developed method is adequate to be applied in the clinical analysis.






Similar content being viewed by others
References
Stoica L, Lindgren-Sjolander A, Ruzgas T, Gorton L (2004) Anal Chem 76:4690
Erdogan H, Tuncagil S, Toppare L (2010) J Macromol Sci A 47:209
Robinson DL, Hermans A, Seipel AT, Wightman RM (2008) Chem Rev 108:2554
Venton BJ, Wightman RM (2003) Anal Chem 75:414A
Lee JM, Xu G-R, Kim BK, Choi HN, Lee W-Y (2011) Electroanalysis 23:962
Piao Y, Jin Z, Lee D, Lee HJ, Na HB, Hyeon T, Oh MK, Kim J, Kim HS (2011) Biosens Bioelectron 26:3192
Song W, Li D-W, Li Y-T, Li Y, Long Y-T (2011) Biosens Bioelectron 26:3181
Lu L, Zhang L, Zhang X, Huan S, Shen G, Yu R (2010) Anal Chim Acta 665:146
Campuzano S, Serra B, Pedrero M, de Villena FJM, Pingarron JM (2003) Anal Chim Acta 494:187
Wang L, Ran Q, Tian Y, Ye S, Xu J, Xian Y, Peng R, Jin L (2010) Microchim Acta 171:217
Kalachar HCB, Basavanna S, Viswanatha R, Naik YA, Raj DA, Sudhad PN (2011) Electroanalysis 23:1107
Sima VH, Patris S, Aydogmus Z, Sarakbi A, Sandulescu R, Kauffmann JM (2011) Talanta 83:980
Notsu H, Tatsuma T (2004) J Electroanal Chem 566:379
Xue HG, Shen ZQ (2002) Talanta 57:289
Zejli H, Hidalgo–Hidalgo de Cisneros JL, Naranjo-Rodriguez I, Liu B, Temsamani KR, Marty JL (2008) Anal Chim Acta 612:198
Marin-Zamora ME, Rojas-Melgarejo F, Garcia-Canovas F, Garcia-Ruiz PA (2005) J Chem Technol Biotechnol 80:1356
Tembe S, Karve M, Inamdar S, Haram S, Melo J, D’Souza SF (2006) Anal Biochem 349:72
Sheldon RA (2007) Adv Synth Catal 349:1289
Pingarron JM, Yanez-Sedeno P, Gonzalez-Cortes A (2008) Electrochim Acta 53:5848
Liu SQ, Ju HX (2003) Biosens Bioelectron 19:177
Liu SQ, Yu JH, Ju HX (2003) J Electroanal Chem 540:61
Manso J, Mena ML, Yanez-Sedeno P, Pingarron J (2007) J Electroanal Chem 603:1
Viswanathan S, Liao W-C, Huang C-C, Hsu W-L, Ho JA (2007) Talanta 74:229
Krishnamoorthy K, Zoski CG (2005) Anal Chem 77:5068
Shi HB, Xia T, Nel AE, Yeh JI (2007) Nanomedicine 2:599
Shi HB, Yeh JI (2007) Nanomedicine 2:587
Prabhu P, Suresh Babu R, Sriman Narayanan S (2011) Sens Actuators B 156:606
Hu GZ, Chen L, Guo Y, Wang XL, Shao SJ (2010) Electrochim Acta 55:4711
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pinho, A., Viswanathan, S., Ribeiro, S. et al. Electroanalysis of urinary l-dopa using tyrosinase immobilized on gold nanoelectrode ensembles. J Appl Electrochem 42, 131–137 (2012). https://doi.org/10.1007/s10800-012-0379-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-012-0379-3