Abstract
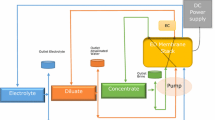
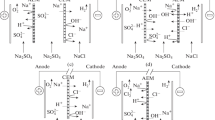
In this work, the chloride behavior through an electrochemical treatment of brines is examined using ion exchange membranes like in electrodialysis. All experiments have been performed using solutions of NaCl before an application on real brines issued from an Algerian desalination plant. After checking oxidation parameters of chloride oxidation by electrolysis, ion exchange membrane have been introduced to control both the pH and the species migrated. The effect of current density and the membrane nature has been studied. The electrochemical treatment described in this work allows transforming the brines in useful products as NaOH, HCl and Cl2. The pH and the salt concentration are varied and the products obtained at the electrodes were identified and analyzed. It was shown that we can get chlorates according to the current density applied and the fixed pH. This fact gives rise to an economical process where valuable products can be obtained using only the chloride oxidation current. Results were linked to the Pourbaix diagram and allow the prediction of the process efficiency.







Similar content being viewed by others
References
Hasson D, Lumelsky V, Greenberg G, Pinhas Y, Semiat R (2008) Desalination 230:329
Nair MKV, Misra BM (1978) Desalination 27:59
Zeppenfeld K (1998) GWF Gas-Wasserfach Wasser/Abwasser 139(2):86
Zeppenfeld K (2000) Chemie Ingenieur Technik 72(1–2):101
Shpiz LL, Yu E, Kirshina Y, Gutnikova RI (1999) Uzbekistan Uzb Khim Zh 4:5
Zabolotskii VI, Reprintseva SL, Gnusin NP (1981) Zh Prikl Khim (Leningrad) 54(6):1345
Deslouis C, Frateur I, Maurin G, Tribollet B (1997) J Appl Electrochem 27(4):482
Gabrielli C, Jaouhari R, Joiret G, Maurin G, Rousseau P (2003) J Electrochem Soc 150(7):478
Devos O, Gabrielli C, Tlili MM, Tribollet B (2003) J Electrochem Soc 150(7):494
Tlili MM, Ben Amor M, Gabrielli C, Joiret S, Maurin G, Rousseau P (2003) J Electrochem Soc 150(7):485
Tlili MM, Ben Amor M, Gabrielli C, Tribollet B (2003) J Electrochem Soc 150(11):765
Van Hegea K, Verhaegeb M, Verstraetea W (2004) Water Res 38:1550
Chiang LC, Chang JE, Wen TC (1995) Water Res 29:671
Wang P, Lau IWC, Fang HHP (2001) Environ Technol 22:373
Vlyssides AG, Papaioannou D, Loizidoy M, Karlis PK, Zorpas AA (2000) Waste Manage 20:569
Panizza M, Bocca C, Cerisola G (2000) Water Res 34:2601
Lin SH, Shyu CT, Sun MC (1998) Water Res 32:1059
Maxhobandile S (2005) Master thesis, University of the Western Cape
Acknowledgment
This work has been granted through the project “TASSILI MDU 699” as a cooperation between the USTHB (Algeria) and the IEM (Montpellier).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naimi, M., Innocent, C. & Akretche, D.E. Chloride behaviour in electromembrane treatment of brine issued from desalination plants. J Appl Electrochem 40, 1079–1083 (2010). https://doi.org/10.1007/s10800-010-0070-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-010-0070-5