Abstract
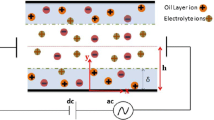
AC electroosmotic micropumps are suggested to be powerful tools for electrolyte dosing in various micro- and nanofluidic systems. In this paper, we compare two modeling approaches for studying the AC electroosmosis in the following micro and nanochannel systems: (i) a traveling-wave AC pump with a spatially continuous wave of electric potential applied on a planar boundary, (ii) a traveling-wave AC pump with a wave of electric potential applied on a set of discrete planar electrodes, and (iii) an AC pump with a set of non-planar electrodes. The equilibrium approach is based on the use of capacitor–resistor boundary conditions for electric potential and the slip boundary conditions for velocity at electrode surfaces. The non-equilibrium approach uses the mathematical model based on the Poisson equation and the non-slip boundary conditions. We have observed discrepancies between the predictions given by the both models and then we have identified their possible reasons. The comparison of the equilibrium and non-equilibrium results further showed three important actualities: (a) how the equilibrium model overestimates or underestimates the net velocity, (b) how the velocity maxima in the frequency characteristics can be shifted, if the equilibrium model assumptions are not satisfied, (c) the parametric region where the equilibrium model is applicable. Because the data are obtained in a dimensionless form, they can be exploited for AC electroosmotic studies. We discuss the limitations of the equilibrium and non-equilibrium models and compare selected predictions with available experimental data.













Similar content being viewed by others
Abbreviations
- A :
-
Amplitude (V)
- c :
-
Concentration (mol m−3)
- C D :
-
Capacitance of EDL (F m−2) \(C_{D} = \varepsilon/\lambda_{D}\)
- D :
-
Diffusivity (2 × 10−9 m2 s−1)
- f :
-
Frequency (s−1)
- F :
-
The Faraday constant (96,485 C mol−1)
- g :
-
Gap width (m) g = x Lm+1 −x R m
- h :
-
Electrode height (m)
- H :
-
Height of a periodic segment (m)
- J :
-
Ion flux intensity (mol m−2s−1)
- k :
-
Wave number (m−1) k = 2π/L
- L :
-
Length of a periodic segment (m)
- L e :
-
Electrode width (m) L e = x R m − x L m
- n :
-
Number of electrodes
- n Fx :
-
Number of finite elements in the x-direction
- n Fy :
-
Number of finite elements in the y-direction
- n :
-
Normal unit vector
- p :
-
Pressure (Pa)
- q :
-
Electric charge density (C m−3)
- R :
-
Molar gas constant (8.314 J K−1mol−1)
- t :
-
Time (s)
- t :
-
Tangential unit vector
- T :
-
Temperature (298.15 K)
- T t :
-
Period of the electric signal (s) T t = f −1
- v :
-
Horizontal component of velocity (m s−1)
- 〈v 〉:
-
Net velocity (m s−1)
- v :
-
Velocity (m s−1)
- w :
-
Electric potential wave velocity (m s−1) w = L/T t = ω/k
- x :
-
Spatial coordinate (m)
- y :
-
Spatial coordinate (m)
- α:
-
Phase of an AC signal
- \(\varepsilon\) :
-
Electrolyte permitivity (6.9503 × 10−10 F m−1)
- φ:
-
Electric potential (V)
- η:
-
Dynamic viscosity (0.001 Pa s)
- λ D :
-
The Debye length (m) \(\lambda_{D}^{2}=\frac{\varepsilon D}{\sigma}\)
- ψ:
-
Complex electric potential (V)
- ρ:
-
Density (1,000 kg m−3)
- σ:
-
Specific conductivity (S m−1) \({\sigma}=2 c_{\circ} D \frac{F^{2}}{RT}\)
- ω:
-
Angular frequency (s−1) ω = 2 πf
- Ra:
-
The Rayleigh number \(\hbox{Ra} = \frac{\varepsilon}{\eta D}\left(\frac{RT}{F}\right)^{2}= 0.2294\)
- Sc:
-
The Schmidt number \(\hbox{Sc} =\frac{\eta}{\rho D} =500\)
- \({\tilde{\lambda}}_{D}\) :
-
EDL simplex \({\tilde{\lambda}}_{D} =\lambda_{D}/L\)
- *:
-
Complex conjugate
- ∼:
-
Dimensionless
- ∧:
-
Time averaged
- +:
-
Cation
- −:
-
Anion
- ±:
-
Either + or −
- e :
-
Electrode
- L :
-
Left boundary of the electrode
- R :
-
Right boundary of the electrode
- C :
-
Center of the electrode
- o:
-
Characteristic value
- m :
-
Index of electrode
- slip:
-
At the slip plane
References
Ramos A, Morgan H, Green NG et al (1998) J Phys D 31:2338
Ajdari A (2000) Phys Rev E 61:R45
Campisi M, Accoto D, Dario P (2005) J Chem Phys 123:204724
Garcia-Sanchez P, Ramos A, Green G et al (2006) IEEE Trans Dielectr Electr Insul 13:670
Green NG, Ramos A, Gonzalez A et al (2002) Phys Rev E 66:026305
Mpholo M, Smith CG, Brown ABD (2003) Sens Actuators B 92:262
Studer V, Pepin A, Chen Y et al (2004) Analyst 129:944
Probstein RF (1994) Physicochemical hydrodynamics: an introduction. Wiley, New York
Green NG, Ramos A, Morgan H (2000) J Phys D 33:632
Bazant MZ, Ben YX (2006) Lab Chip 6:1455
Burch D, Bazant MZ (2008) Phys Rev E 77:055303(R)
Urbanski JP, Thorsen T, Levitan JA et al (2006) Appl Phys Lett 89:143508
Urbanski JP, Levitan JA, Burch DN et al (2007) J Colloid Interface Sci 309:332
Cahill BP, Heyderman LJ, Gobrecht J et al (2004) Phys Rev E 70:036305
Cahill BP, Heyderman LJ, Gobrecht J et al (2005) Sens Actuators B 110:157
Ramos A, Gonzalez A, Garcia-Sanchez P et al (2007) J Colloid Interface Sci 309:323
Ejsing L, Smistrup K, Pedersen CM et al (2006) Phys Rev E 73:037302
Mortensen NA, Olesen LH, Belmon L et al (2005) Phys Rev E 71:056306
Ramos A, Morgan H, Green NG et al (2005) J Appl Phys 97:084906
Squires TM, Bazant MZ (2004) J Fluid Mech 509:217
Olesen LH, Bruus H, Ajdari A (2006) Phys Rev E 73:056313
Kim BJ, Yoon SY, Sung H J et al (2007) J Appl Phys 102:074513
Loucaides N, Ramos A, Georghiou GE (2007) Microfluid Nanofluid 3:709
Khan T, Reppert PM (2005) J Colloid Interface Sci 290:574
Wang XM, Wu JK (2006) J Colloid Interface Sci 293:483
Pribyl M, Snita D, Marek M (2008) Multiphysical modeling of DC and AC electroosmosis in micro- and nanosystems. In: Petrone G, Cammarata G (eds) Recent advances in modelling and simulation. I-Tech Education and Publishing, Vienna
Cervenka P, Pribyl M, Snita D (2009) Microelectron Eng 86:1333
Kilic MS, Bazant MZ, Ajdari A (2007) Phys Rev E 75:021502
Kilic MS, Bazant MZ, Ajdari A (2007) Phys Rev E 75:021503
Storey BD, Edwards L R, Kilic MS et al (2008) Phys Rev E 77:036317
Levitan JA, Devasenathipathy S, Studer V et al (2005) Colloids Surf A 267:122
González A, Ramos A, Green NG et al (2000) Phys Rev E 61:4019
Postler T, Slouka Z, Svoboda M et al (2008) J Colloid Interface Sci 320:321
Deen WM (1998) Analysis of transport phenomena. Oxford University Press, New York
Acknowledgements
The authors thank for the support by the grant of the GAAV ČR (KAN208240651), by the grant of the MŠMT ČR (MSM 6046137306), by the grant MPO ČR (Pokrok 1H-PK/24), and by the grant GAČR (GD 104/08/H055).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hrdlička, J., Červenka, P., Přibyl, M. et al. Mathematical modeling of AC electroosmosis in microfluidic and nanofluidic chips using equilibrium and non-equilibrium approaches. J Appl Electrochem 40, 967–980 (2010). https://doi.org/10.1007/s10800-009-9966-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-009-9966-3