Abstract
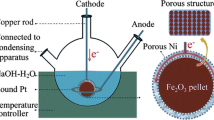
Iron(III) oxide tablets were electrolytically reduced to iron in molten sodium hydroxide at 530 °C and recovered to produce iron with 2 wt.% oxygen suitable for re-melting. The cell was operated at 1.7 V and an inert nickel anode was used. The thermodynamics and mechanism of the process was also investigated. By controlling the activity of sodium oxide in the melt, the cell could be operated below the decomposition voltage of the electrolyte with the net sequence of events being the ionization of oxygen, its subsequent transport to the anode and discharge leaving behind iron at the cathode. A reduction time of 1 h was achieved for a 1 g oxide tablet (close to the theoretical reduction time predicted by Faraday’s laws) at a current density of 520 mA cm−2 with iron phase yields of ∼90 wt.%. The energy consumption was 2.8 kWh kg−1.




Similar content being viewed by others
References
Industries (2006) E.C.o.I.a.S
Chen GZ, Fray DJ, Farthing TW (2000) Nature 407:361–364
Burheim OS (2005) Institutt for Material Teknologi, University of Trondheim
Plambeck JA (1976) Encyclopedia of electrochemistry of the elements. Marcel Dekker
Newell LC (1904) Descriptive chemistry. Heath
Tremillon B (1971) Pure Appl Chem 25:395
Schwandt C, Fray DJ (2005) Electrochimica Acta 51:66–76
Yan XY, Fray DJ (2005) J Electrochem Soc 152:D12–D21
Bouaziz R, Papin G, Rollet AP (1966) C R Acad Sci Ser C 262:1053
Acknowledgements
The authors are grateful to the EPSRC for funding (Platform Grant GR/S58447/01) the work in this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cox, A., Fray, D.J. Mechanistic investigation into the electrolytic formation of iron from iron(III) oxide in molten sodium hydroxide. J Appl Electrochem 38, 1401–1407 (2008). https://doi.org/10.1007/s10800-008-9579-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-008-9579-2