Abstract
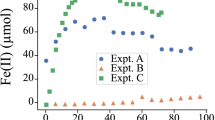
As(V) is electrochemically reduced on the surface of a carbonaceous electrode in the presence of hematite and hydrochloric acid 1 M. The influence of other iron oxides (goethite and limonite) was also tested, although they did not provided better results. The potential required to achieve the reduction must be lower than −0.3 V. The anodic voltammograms exhibit a peak at 0.14 V which corresponds to the oxidation of the As° previously generated during the pre-treatment step (−1 V, 5 s) to As(III). Fe(II) generated during the pre-treatment step plays a relevant role in the final reduction to As° which is subsequently reoxidized to As(III) in the anodic scan. This has been applied to the direct detection of 5 mg kg−1 of arsenic in a solid sample of compost with high concentration of iron oxides by square wave voltammetry.



Similar content being viewed by others
References
Wei Z, Somasundaran P (2004) J Appl Electrochem 34:241
Kao WH, Kuwana T (1984) J Electroanal Chem 169:167
Eguiarte I, Alonso KM, Jiménez RM (1996) Analyst 121:1835
Greulach U, Henze G (1995) Anal Chim Acta 306:217
Li H, Smart RB (1996) Anal Chim Acta 325:25
He Y, Zheng Y, Locke DC (2007) Microchem J 85:265
Forsberg G, O’lauglin JW, Megargle RG, Koirtyohann SR (1975) Anal Chem 47:1586
Feeney R, Kounaves SP (2002) Talanta 58:23
Huang H, Dasgupta PK (1999) Anal Chim Acta 380:27
Huiliang H, Jagner D, Renman L (1988) Anal Chim Acta 207:37
Gründler P, Flechsig GU (1998) Electrochim Acta 433:451
Schickling C, Yang JF, Broekaert JAC (1996) J Anal At Spectrom 11:739
Bose P, Sharma A (2002) Water Res 36:4916
Bauer M, Blodau C (2006) Sci Total Environ 354:179
Lin H, Wang MC, Li GC (2004) Chemosphere 46:1105
Melitas N, Conklin M, Farrel J (2002) Environ Sci Technol 36:3188
Locatelli C, Torsi G (2001) J Electroanal Chem 509:80
Locatelli C, Torsi G (2000) Microchem J 65: 293
Scholz F, Schröder U, Gulaboski R (2005) Electrochemistry of immobilized particles and droplets. Springer-Verlag, Berlin-Heidelberg
Cepriá G, Alexa N, Cordos E, Castillo JR (2005) Talanta 66:875
Schwertmann U, Cornell RM (1991) Iron oxides in the laboratory VCH. Weinheim
Grygar T (1995) Collect Czech Chem Commun 60:1261
Su C, Puls R (2001) Environ Sci Technol 35:1487
Acknowledgement
This work was supported by the SGPCCC of the Spanish Ministry of the Environment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cepriá, G., Hamida, S., Laborda, F. et al. Direct reduction of As(V) physically attached to a graphite electrode mediated by Fe(III). J Appl Electrochem 37, 1171–1176 (2007). https://doi.org/10.1007/s10800-007-9380-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-007-9380-7