Abstract
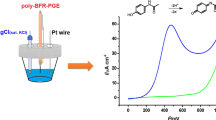
Prussian Blue-modified graphite electrodes (G/PB) with electrocatalytic activity toward H2O2 reduction were obtained by PB potentiostatic electrodeposition from a mixture containing 2.5 mm FeCl3 + 2.5 mm K3[Fe(CN)6] + 0.1 m KCl + 0.1 m HCl. From cyclic voltammetric measurements, performed in KCl aqueous solutions of different concentrations (5 × 10−2–1 m), the rate constant for the heterogeneous electron transfer (k s) was estimated by using the Laviron treatment. The highest ks value (10.7 s−1) was found for 1 m KCl solution. The differences between the electrochemical parameters of the voltammetric response, as well as the shift of the formal potential, observed in the presence of Cl− and NO −3 compared to those observed in the presence of SO 2−4 ions, points to the involvement of anions in the redox reactions of PB. The G/PB electrodes showed a good electrochemical stability proved by a low deactivation rate constant (0.8 × 10−12 mol cm2 s−1). The electrocatalytic efficiency, estimated as the ratio \((I_{cat})_{H_2 O_2 } /(I_{cat})\), was found to be 3.6 (at an applied potential of 0 mV vs. SCE; Γ = 5 × 10−8 mol cm−2) for a H2O2 concentration of 5 mm, thus indicating G/PB electrodes as possible H2O2 sensors.





Similar content being viewed by others
References
Karyakin A.A., Karyakina E.E., Gorton L. (1999). Electrochem. Commun. 1:78
Koncki R. (2002). Anal. Chem. 32:79
Karyakin A.A., Karyakina E.E., Gorton L. (1998). J. Electroanal. Chem. 456:97
Feldman B.J., Melroy O.R. (1987). J. Electroanal. Chem. 234:213
Keggin J.F., Miles F.D. (1936). Nature 137:577
Ellis D., Eckhoff M., Neff V.D.(1981). J. Phys. Chem. 85:1225
Itaya K., Ataka T., Toshima S.(1982). J. Am. Chem. Soc. 104:4767
Garcia Jareno J.J., Navarro-Laboulais J., Vicente F. (1996). Electrochim. Acta 41:835
Karyakin A.(2001). Electroanalysis 13:813
Huck H.(1999). Phys. Chem. Chem. Phys. 1:855
Murray R.W. (1984). In: Bard A.J. (ed.) Electroanalytical Chemistry. Marcel Dekker, New York, pp. 191
Gorton L.(1995). Electroanalysis 7:23
Kellawi H., Rosseinsky D.R. (1982). J. Electrochem. Soc.131:373
Murray R.W. (1992). In: Murray R.W. (ed.) Molecular Design of Electrode Surfaces. J. Wiley New York, pp. 1
Laviron E. (1979). J. Electroanal. Chem. 101:19
Honeychurch M.J., Rechnitz G.A. (1998). Electroanalysis 5:285
Malik M.A., Horanyi G., Kulesza P.J., Inzelt G., Kertesz V., Schmidt R., Czirok E. (1998). J. Electroanal. Chem. 452:57
Feldman B.J., Murray R.W. (1987). Inorg. Chem. 26:1702
Acknowledgment
Financial support from CNCSIS (Grants Nr. 51/349 /2005 and TD 6/89-2005) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cretu, R.C., Gligor, D.M., Muresan, L. et al. Kinetic characterization of Prussian Blue-modified graphite electrodes for amperometric detection of hydrogen peroxide. J Appl Electrochem 36, 1327–1332 (2006). https://doi.org/10.1007/s10800-006-9242-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-006-9242-8