Abstract
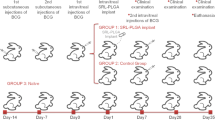
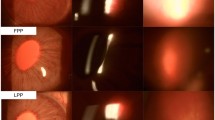
Conventional treatments of uveitis are not ideal because of the short period of therapeutic efficacy. In the present study, biodegradable polylactic-glycolic acid microspheres loaded with triamcinolone acetonide (TA) were prepared to achieve sustained drug release and their therapeutic efficacy was investigated on a rabbit model of uveitis. TA-loaded microspheres (TA-MS) were prepared by the solvent evaporation method and characterized for encapsulation efficiency, particle size, morphology and in vitro release. The therapeutic efficacy was studied on the rabbit experimental uveitis model based on scoring of the inflammation, aqueous leukocyte counting, aqueous protein determination and histological examination. The TA-MS exhibited smooth and intact surfaces with an average diameter of 50.87 μm. The drug-loading coefficient and encapsulation efficiency were 15.2 ± 0.6 % and 91.24 ± 3.77 %, respectively. The drug release from TA-MS lasted up to 87 days, but only 46 days for TA suspension. The change in surface morphology also showed sustained drug release from TA-MS. TA-MS exhibited improved therapeutic efficacy in lipopolysaccharide -induced uveitis compared to TA suspension, especially in regard to the inhibition of inflammation. The TA-MS had a longer-term therapeutic effect on intraocular inflammation in LPS-induced uveitis in rabbits compared to TA suspension. The results suggested that TA-MS can be developed as a potential sustained-release system for the treatment of uveitis.




Similar content being viewed by others
Abbreviations
- TA:
-
Triamcinolone acetonide
- MS:
-
Microspheres
- TA-MS:
-
TA-loaded microshperes
- PLGA:
-
Polylactic-glycolic acid
- LPS:
-
Lipopolysaccharide
- AS:
-
Aseptic saline
- EIU:
-
Endotoxin-induced uveitis
- SEM:
-
Scanning electron microscopy
- PVA:
-
Polyvinyl alcohol
- MS-blank:
-
TA-unloaded microspheres
- HPLC:
-
High-performance liquid chromatography
- EE:
-
Encapsulation efficiency
- PBS:
-
Phosphate-buffered saline
References
Jabs DA, Nussenblatt RB, Rosenbaum JT (2005) Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 140(3):509–516
Suttorp-Schulten MS, Rothova A (1996) The possible impact of uveitis in blindness: a literature survey. Br J Ophthalmol 80(9):844–848
Smith JR (2004) Management of uveitis. Clin Exp Med 4(1):21–29
Chin HS, Park TS, Moon YS et al (2005) Difference in clearance of intravitreal triamcinolone acetonide between vitrectomized and non-vitrectomized eyes. Retina 25(5):556–560
Martidis A, Duker JS, Greenberg PB et al (2002) Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology 109(5):920–927
Cunningham MA, Edelman JL, Kaushal S (2008) Intravitreal steroids for macular edema: the past, the present, and the future. Surv Ophthalmol 53(2):139–149
Spaide RF, Sorenson J, Maranan L (2003) Combined photodynamic therapy with verteporfin and intravitreal triamcinolone acetonide for choroidal neovascularization. Ophthalmology 110(8):1517–1525
Kok H, Lau C, Maycock N et al (2005) Outcome of intravitreal triamcinolone in uveitis. Ophthalmology 112(11):1916 e1911–1917
Gilger BC, Malok E, Stewart T et al (2000) Long-term effect on the equine eye of an intravitreal device used for sustained release of cyclosporine A. Vet Ophthalmol 3(2–3):105–110
Jermak CM, Dellacroce JT, Heffez J et al (2007) Triamcinolone acetonide in ocular therapeutics. Surv Ophthalmol 52(5):503–522
Salvolini E, Neri P, Orciani M, Di Primio R, Giovannini A (2008) Intravitreal micronized triamcinolone versus triamcinolone acetonide: a clinical and morphological comparative study. Int J Immunopathol Pharmacol 21(1):181–188
Haghjou N, Soheilian M, Abdekhodaie MJ (2011) Sustained release intraocular drug delivery devices for treatment of uveitis. J Ophthalmic Vis Res 6(4):317–329
Delie F, Blanco-Prieto MJ (2005) Polymeric particulates to improve oral bioavailability of peptide drugs. Molecules 10(1):65–80
Barratt GM (2000) Therapeutic applications of colloidal drug carriers. Pharm Sci Technol Today 3(5):163–171
Washington C (1996) Drug release from microparticulate systems. In: Benita S (ed) Microencapsulation: methods and industrial applications. Marcel Dekker, New York, pp 155–181
Sy JC, Davis ME (2010) Delivering regenerative cues to the heart: cardiac drug delivery by microspheres and peptide nanofibers. J Cardiovasc Transl Res 3(5):461–468
Bhardwaj U, Papadimitrakopoulos F, Burgess DJ (2008) A review of the development of a vehicle for localized and controlled drug delivery for implantable biosensors. J Diabetes Sci Technol 2(6):1016–1029
Mansoor S, Kuppermann BD, Kenney MC (2009) Intraocular sustained-release delivery systems for triamcinolone acetonide. Pharm Res 26(4):770–784
Herrero-Vanrell R, Refojo MF (2001) Biodegradable microspheres for vitreoretinal drug delivery. Adv Drug Deliv Rev 52(1):5–16
Vallelado AI, Lopez MI, Calonge M et al (2002) Efficacy and safety of microspheres of cyclosporin A, a new systemic formulation, to prevent corneal graft rejection in rats. Curr Eye Res 24(1):39–45
Khaled KA, Sarhan HA, Ibrahim MA et al (2010) Prednisolone-loaded PLGA microspheres. in vitro characterization and in vivo application in adjuvant-induced arthritis in mice. AAPS Pharm Sci Tech 11(2):859–869
Koga T, Koshiyama Y, Gotoh T et al (2002) Coinduction of nitric oxide synthase and arginine metabolic enzymes in endotoxin-induced uveitis rats. Exp Eye Res 75(6):659–667
Hanashiro RK, Fujino Y, Gugunfu et al (1997) Synthetic lipid A-induced uveitis and endotoxin-induced uveitis—a comparative study. Jpn J Ophthalmol 41(6):355–361
Rosenbaum JT, McDevitt HO, Guss RB et al (1980) Endotoxin-induced uveitis in rats as a model for human disease. Nature 286(5773):611–613
Hoekzema R, Murray PI, van Haren MA et al (1991) Analysis of interleukin-6 in endotoxin-induced uveitis. Invest Ophthalmol Vis Sci 32(1):88–95
Cheng CK, Berger AS, Pearson PA et al (1995) Intravitreal sustained-release dexamethasone device in the treatment of experimental uveitis. Invest Ophthalmol Vis Sci 36(2):442–453
Barcia E, Herrero-Vanrell R, Diez A et al (2009) Downregulation of endotoxin-induced uveitis by intravitreal injection of polylactic-glycolic acid (PLGA) microspheres loaded with dexamethasone. Exp Eye Res 89(2):238–245
Cardillo JA, Souza-Filho AA, & Oliveira AG (2006) Intravitreal Bioerudivel sustained-release triamcinolone microspheres system (RETAAC). Preliminary report of its potential usefulnes for the treatment of diabetic macular edema. Arch Soc Esp Oftalmol 81(12):675–677, 679–681
Siepmann J, Faisant N, Akiki J et al (2004) Effect of the size of biodegradable microparticles on drug release: experiment and theory. J Control Release 96(1):123–134
Zarei-Ghanavati S, Malaekeh-Nikouei B, Pourmazar R et al (2012) Preparation, characterization, and in vivo evaluation of triamcinolone acetonide microspheres after intravitreal administration. J Ocul Pharmacol Ther 28(5):502–506
Yasukawa T, Ogura Y, Tabata Y et al (2004) Drug delivery systems for vitreoretinal diseases. Prog Retin Eye Res 23(3):253–281
Herrero-Vanrell R, Ramirez L, Fernandez-Carballido A et al (2000) Biodegradable PLGA microspheres loaded with ganciclovir for intraocular administration. Encapsulation technique, in vitro release profiles, and sterilization process. Pharm Res 17(10):1323–1328
Leeds JM, Henry SP, Truong L et al (1997) Pharmacokinetics of a potential human cytomegalovirus therapeutic, a phosphorothioate oligonucleotide, after intravitreal injection in the rabbit. Drug Metab Dispos 25(8):921–926
Howes EL Jr, Morrison DC (1980) Lipid A dependence of the ocular response to circulating endotoxin in rabbits. Infect Immun 30(3):786–790
Beer PM, Wong SJ, Schartman JP et al (2010) Infliximab stability after reconstitution, dilution, and storage under refrigeration. Retina 30(1):81–84
Acknowledgments
This work was supported by the Program for New Drug R&D (No. 2009ZX09310-001) and Innovation Team of Ministry of Education (No. BMU20110263). The authors thank Dr. Jiying Wang for his critical final manuscript revision.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, W., He, B., Dai, W. et al. Evaluations of therapeutic efficacy of intravitreal injected polylactic-glycolic acid microspheres loaded with triamcinolone acetonide on a rabbit model of uveitis. Int Ophthalmol 34, 465–476 (2014). https://doi.org/10.1007/s10792-013-9829-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-013-9829-0