Abstract
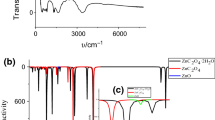
Zinc ammonium phosphate (ZnNH4PO4) was synthesized via a simple precipitation method at room temperature. Thermal decomposition of ZnNH4PO4 occurred through five stages related to the deamination (1st and 2nd steps) and deprotonated hydrogen phosphate (3rd, 4th, 5th steps) reactions and its final decomposed product at above 600 °C was zinc pyrophosphate (to γ-Zn2P2O7). ZnNH4PO4 and γ-Zn2P2O7 samples were analyzed by XRF, XRD, FT-IR and SEM techniques, which are significant for the further treatments. Kinetic parameters (Ea, A) and thermodynamic functions (ΔH*, ΔS* and ΔG*) calculated on well-known equations have been used to support for five thermal transformation processes, reported for the first time. The kinetic results indicate that the intramolecular dehydration of the protonated hydrogen phosphate groups (3rd, 4th, 5th steps) need higher-energy pathways than the deammonium reactions (1st and 2nd steps) because of harder reactions, more difficulties and lower rates. Additionally, thermodynamic results reveal that all thermal reactions are endothermic and non-spontaneous processes. The obtained data may be useful for industrialists and academicians to apply these zinc phosphates for large roles in industrial applications.




Similar content being viewed by others
References
M.H. Debray, Acad. Sci. 59, 40 (1864)
H. Anandalakshmi, K. Velavan, I. Sougandi, R. Venkatesan, P.S. Rao, Pramana 62, 77 (2004)
V.G. Koleva, T.J. Boyadzhieva, R.K. Stoyanova, Cryst. Growth Des. 19, 3744 (2019)
A.F. Holdsworth, H. Eccles, A.M. Halman, R.J. Mao, G. Bond, Sci. Rep. 8, 13547 (2018)
P.H. Peng, W.R. Ernst, G.L. Bridger, E.M. Hartley, Ind. Eng. Chem. Proc. Des. Dev. 18, 453 (1979)
A.W. Frazier, J.P. Smith, J.R. Lehr, J. Agric. Food Chem. 14, 522 (1966)
G.O. Guerrant, D.E. Brown, J. Agric. Food Chem. 13, 493 (1965)
C. Zeng, W. Wei, L. Zhang, CrystEngComm 14, 3008 (2012)
C. Danvirutai, P. Noisong, S. Youngme, J. Therm. Anal. Calorim. 100, 117 (2010)
L.M. Lapina, Russ. Chem. Rev. 37, 693 (1968)
V. Barron, J. Torrent, J. Agric. Food Chem. 42, 105 (1994)
J.E. Greedan, K. Reubenbauer, T. Birchall, M. Ehlert, D.R. Corbin, M.A. Subramanian, J. Solid State Chem. 77, 376 (1988)
M. Shwetha, B. Eraiah, Mater. Sci. Eng. 310, 012033 (2018)
Z. Bircsak, W.T.A. Harrison, Acta Cryst. C 54, 1383 (1998)
M.A. Petrova, V.I. Shitova, G.A. Mikirticheva, V.F. Popova, A.E. Malshikov, J. Solid State Chem. 119, 219 (1995)
R. Baitahe, N. Vittayakorn, Thermochim. Acta 596, 21 (2014)
B. Boonchom, R. Baitahe, S. Kongtaweelert, N. Vittayakorn, Ind. Eng. Chem. Res. 49, 3571 (2010)
L. Guo, C.-M. Sun, G.-Y. Li, C.-P. Liu, C.-N. Ji, J. Hazard. Mater. 161, 510 (2009)
T. Bataille, P. Bénard-Rocherullé, D. Louër, J. Solid State Chem. 140, 62 (1998)
A. Zakeri, R. Yazdani-Rad, M.H. Enayati, M.R. Rahimipour, J. Alloys Comp. 403, 258 (2005)
H.E. Kissinger, Anal. Chem. 29, 1702 (1957)
C. Popescu, Thermochim. Acta 285, 309 (1996)
L. Vlaev, N. Nedelchev, K. Gyurova, M. Zagorcheva, J. Anal. Appl. Pyrol. 81, 253 (2008)
V. Mamleev, S. Bourbigot, M. Le Bras, S. Duquesne, J. Sestak, Phys. Chem. Chem. Phys. 2, 4708 (2000)
H.F. Cordes, J. Phys. Chem. 72, 2185 (1968)
S.C. Turmanova, S.D. Genieva, A.S. Dimitrova, L.T. Vlaev, Express Polym. Lett. 2, 133 (2008)
J.M. Criado, L.A. Pérez-Maqueda, P.E. Sánchez-Jiménez, J. Therm. Anal. Calorim. 82, 671 (2005)
D. Brandová, M. Trojan, M. Arnold, F. Paulik, J. Paulik, J. Therm. Anal. 34, 1449 (1988)
C. Sronsri, B. Boonchom, J. Therm. Anal. Calorim. 134, 1575 (2018)
G. Zhan, J.-X. Yu, Z.-G. Xu, F. Zhou, R.-A. Chi, Trans. Nonferr. Metals Soc. China 22, 925 (2012)
W. Wenwei, W. Xuehang, L. Shuibin, L. Sen, J. Therm. Anal. Calorim. 104, 685 (2010)
B.D. Cullity, Elements of X-ray diffraction, vol. 2 (Addison Wesley Publishing, Boston, 1977)
G. Berhault, P. Afanasiev, H. Loboue, C. Geantet, T. Cseri, C. Pichon, C. Guillot-Deudon, A. Lafond, Inorg. Chem. 48, 2985 (2009)
B. Boonchom, R. Baitahe, Mater. Lett. 63, 2218 (2009)
A. Zecchina, L. Marchese, S. Bordiga, C. Pazè, E. Gianotti, J. Phys. Chem. B 101, 10128 (1997)
N. Kongkaew, W. Pruksakit, S. Patumsawad, Energy Procedia 79, 663 (2015)
Ö. Çepelioğullar, H. Haykırı-Açma, S. Yaman, Waste Manag. 48, 275 (2016)
C. Head, A.C.K. Smith, Applied physical chemistry (McMilan Press, London, 1982), p. 473
J.J. Ŝesták, Thermodynamical properties of solids (Academia, Prague, 1984)
D. Young, Decomposition of solids (Pergamon Press, Oxford, 1966)
Acknowledgements
This work is supported by King Mongkut’s Institute of Technology Ladkrabang [KREF146002].
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baitahe, R., Boonchom, B. A Simple Synthesis, Characterization, Kinetics and Thermodynamics of Zinc Ammonium Phosphate, ZnNH4PO4. Int J Thermophys 41, 33 (2020). https://doi.org/10.1007/s10765-020-2610-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-020-2610-5